Abstract
1 RS-15385-197 ((8aR, 12aS, 13aS)-5,8,8a,9,10,11,12,12a,13,13a-decahydro-3-methoxy-12-(methylsul- phonyl)-6H-isoquino [2,1-g][1,6]-naphthyridine) was evaluated in a series of in vitro and in vivo tests as an antagonist at α2-adrenoceptors.
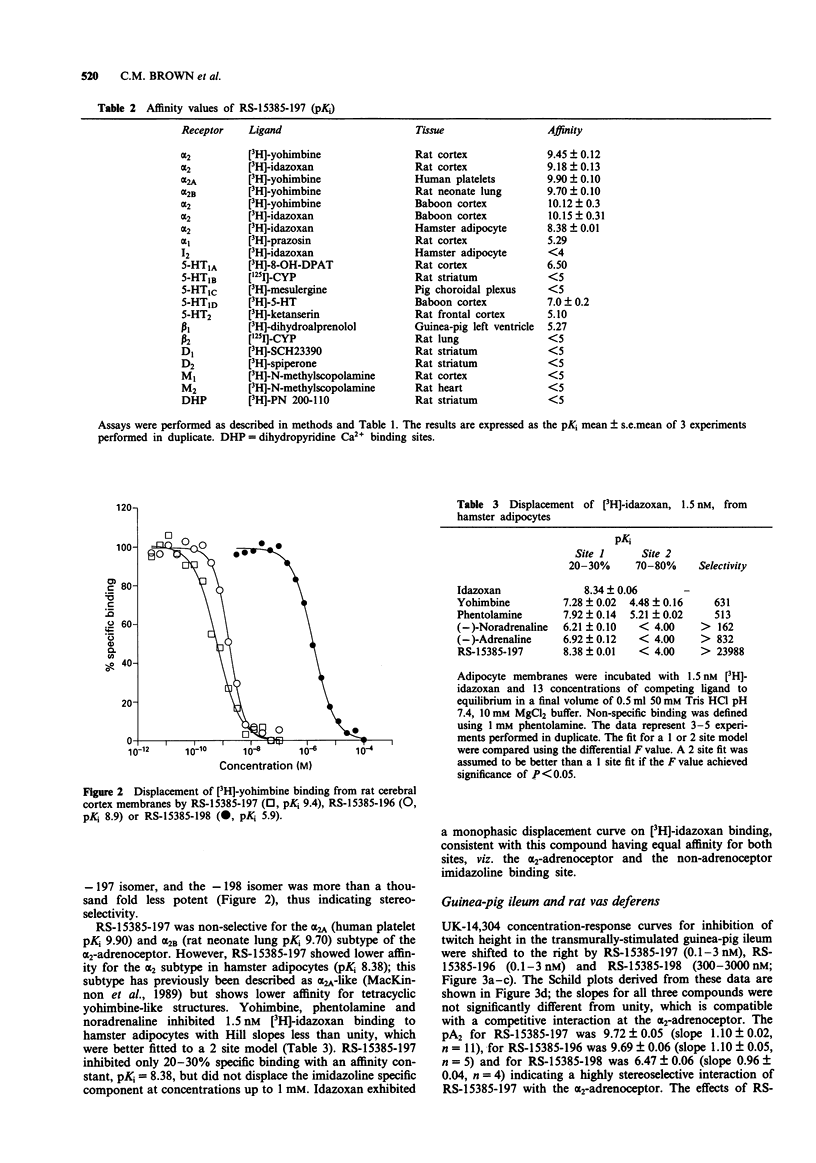
2 RS-15385-197 had a pKi of 9.45 for α2-adrenoceptors in the rat cortex (pA2 in the guinea-pig ileum of 9.72), whereas the 8aS, 12aR, 13aR enantiomer, RS-15385-198, had a pKi of only 6.32 (pA2 6.47) indicating a high degree of stereoselectivity. The racemate RS-15385-196 had a pKi of 9.18.
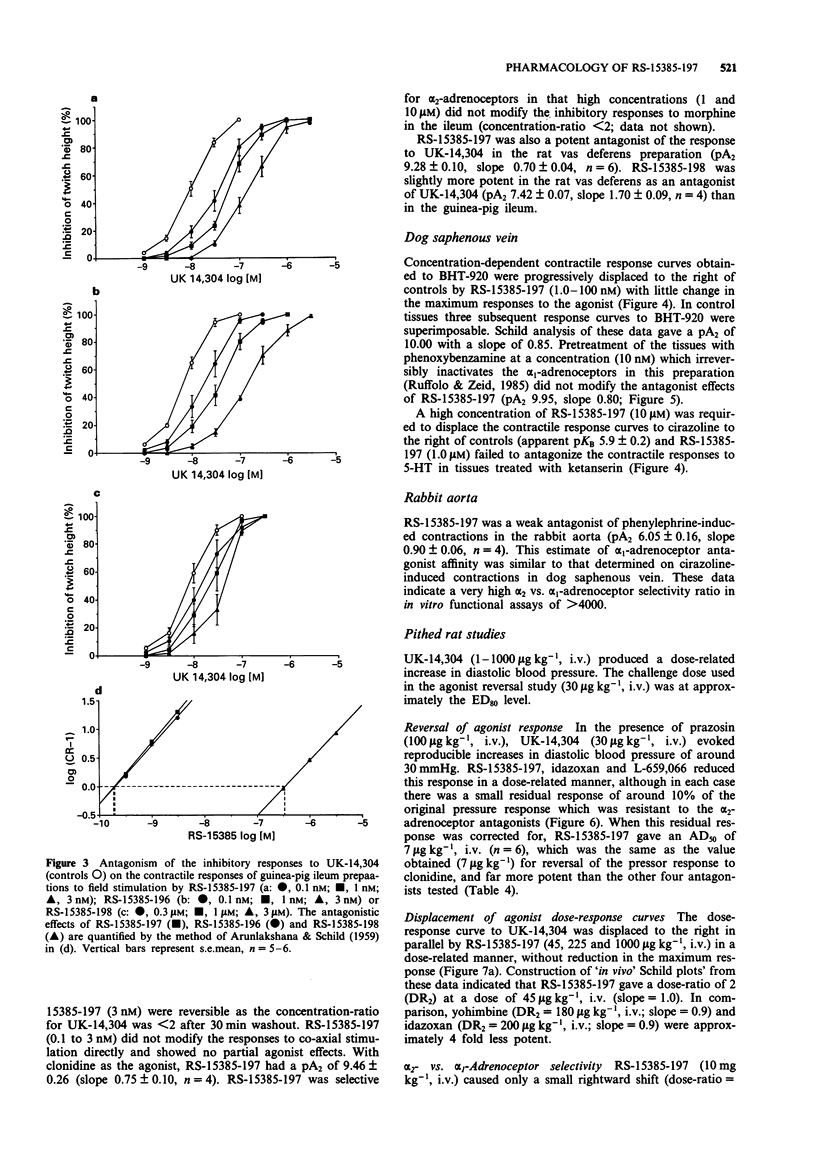
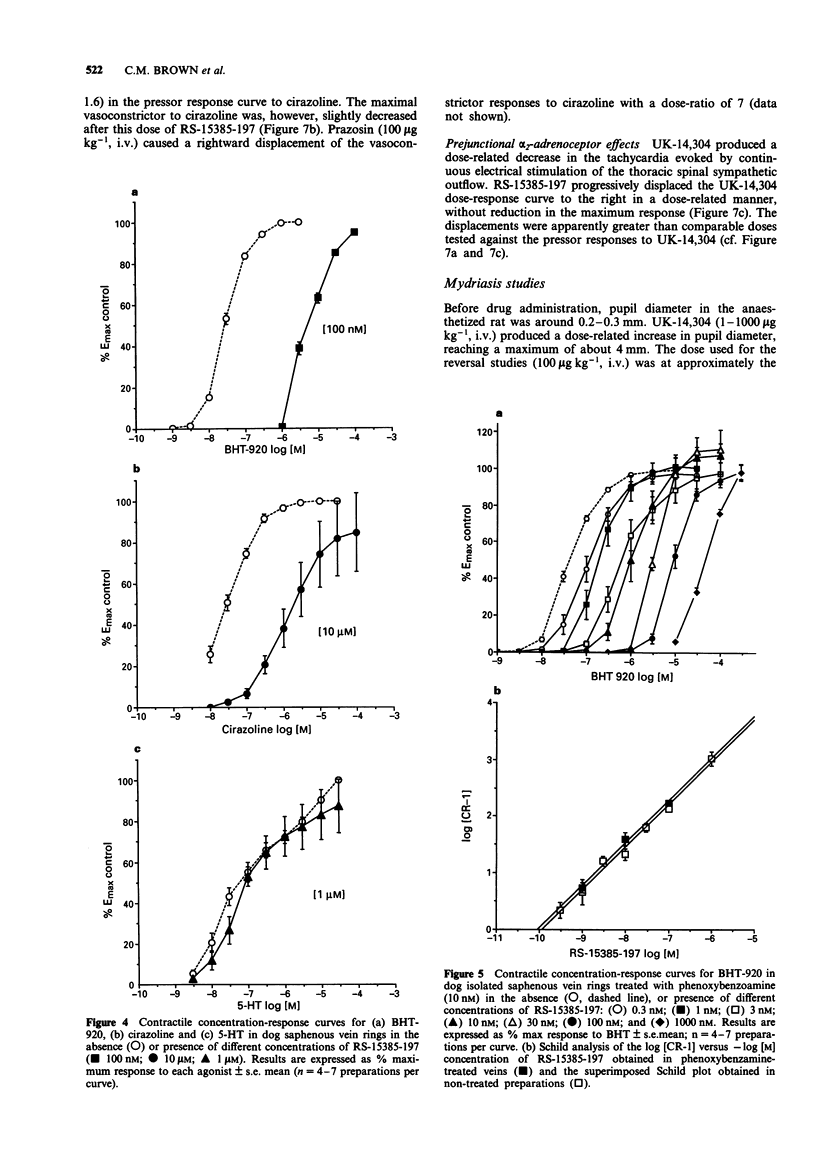
3 RS-15385-197 showed unprecedented α2 vs. α1 adrenoceptor selectivity in vitro. In the rat cortex, RS-15385-197 had a pKi of 9.45 in displacing [3H]-yohimbine and 5.29 in displacing [3H]-prazosin (α2/α1 selectivity ratio in binding experiments > 14000). The compound had a pA2 of 9.72 as a competitive antagonist of the inhibitory effects of UK-14,304 in transmurally-stimulated guinea-pig ileum and 10.0 against BHT-920-induced contractions in dog saphenous vein (DSV); this latter value was unaltered by phenoxybenzamine. An apparent pKB of 5.9 was obtained against cirazoline-induced contractions in DSV, whilst a pA2 of 6.05 was obtained against phenylephrine-induced contractions in the rabbit aorta (α2/α1 selectivity ratio in functional experiments > 4000).
4 RS-15385-197 was highly selective for α2-adrenoceptors over other receptors: the compound showed low affinity for 5-HT1A (pKi 6.50) and 5-HT1D (pKi 7.00) receptor subtypes, and even lower affinity (pKi≤5) for other 5-HT receptor subtypes, dopamine receptors, muscarinic cholinoceptors, β-adrenoceptors and dihydropyridine binding sites. RS-15385-197 was devoid of affinity for the non-adrenoceptor imidazoline binding site, labelled by [3H]-idazoxan, which provides further evidence that these sites are not related to α2-adrenoceptors. In the DSV, contractile responses to 5-hydroxytryptamine (5-HT) were unaffected by a concentration of 1 μM RS-15385-197.
5 RS-15385-197 was non-selective for the α2A- and α2B-adrenoceptor subtypes in that the pKi for the α2A-adrenoceptor in human platelets was 9.90 and the pKi for the α2B-adrenoceptor in rat neonate lung was 9.70. However, RS-15385-197 showed lower affinity for the α2-adrenoceptor subtype in hamster adipocytes (pKi 8.38).
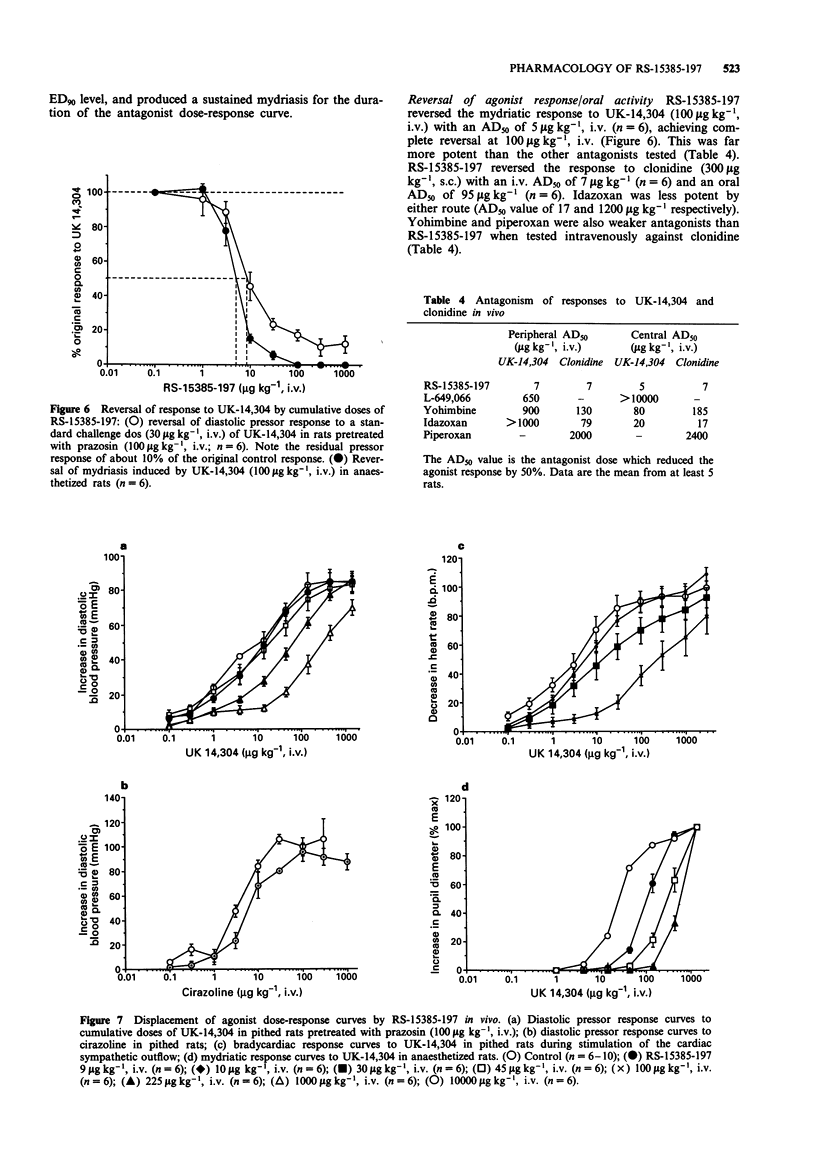
6 In anaesthetized rats, RS-15385-197 was a potent antagonist of the mydriasis response induced by UK-14,304 or clonidine (AD50 5 and 7 μg kg-1, i.v., respectively; 96 μg kg-1, p.o.) and of UK-14,304-induced pressor responses in pithed rats (AD50 7 μg kg-1, i.v.); the compound therefore is both centrally and orally active. Even at a high dose (10 mg kg-1, i.v.), RS-15385-197 did not antagonize pressor responses to cirazoline in pithed rats, indicating that the selectivity for α2 vs. α1-adrenoceptors was maintained in vivo.
8 RS-15385-197 is therefore a very potent, selective, competitive α2-adrenoceptor antagonist, both in vitro and in vivo, is orally active and readily penetrates the brain. It will thus be a powerful pharmacological tool for exploring the various physiological roles of α2-adrenoceptors.
Keywords: RS-15385-197, α2-adrenoceptors, yohimbine, idazoxan
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge T. L., Gadie B., Roach A. G., Tulloch I. F. alpha 2-Adrenoceptor agonists induced mydriasis in the rat by an action within the central nervous system. Br J Pharmacol. 1983 Mar;78(3):507–515. doi: 10.1111/j.1476-5381.1983.tb08810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., MacKinnon A. C., McGrath J. C., Spedding M., Kilpatrick A. T. Alpha 2-adrenoceptor subtypes and imidazoline-like binding sites in the rat brain. Br J Pharmacol. 1990 Apr;99(4):803–809. doi: 10.1111/j.1476-5381.1990.tb13010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., MacKinnon A. C., McGrath J. C., Spedding M., Kilpatrick A. T. Heterogeneity of alpha 2-adrenoceptors in rat cortex but not human platelets can be defined by 8-OH-DPAT, RU 24969 and methysergide. Br J Pharmacol. 1990 Mar;99(3):481–486. doi: 10.1111/j.1476-5381.1990.tb12954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund D. B. Subtypes of alpha 2-adrenoceptors: pharmacological and molecular biological evidence converge. Trends Pharmacol Sci. 1988 Oct;9(10):356–361. doi: 10.1016/0165-6147(88)90254-4. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Clark R. D., Michel A. D., Whiting R. L. Pharmacology and structure-activity relationships of alpha 2-adrenoceptor antagonists. Prog Med Chem. 1986;23:1–39. doi: 10.1016/s0079-6468(08)70339-4. [DOI] [PubMed] [Google Scholar]
- Clark R. D., Repke D. B., Berger J., Nelson J. T., Kilpatrick A. T., Brown C. M., MacKinnon A. C., Clague R. U., Spedding M. Structure-affinity relationships of 12-sulfonyl derivatives of 5,8,8a,9,10,11,12,12a,13,13a-decahydro-6H-isoquino[2,1-g][1 ,6] naphthyridines at alpha-adrenoceptors. J Med Chem. 1991 Feb;34(2):705–717. doi: 10.1021/jm00106a036. [DOI] [PubMed] [Google Scholar]
- Clark R. D., Repke D. B., Kilpatrick A. T., Brown C. M., MacKinnon A. C., Clague R. U., Spedding M. (8a alpha,12a alpha,13a alpha)-5,8,8a,9,10,11,12,12a,13,13a-decahydro- 3-methoxy-12-(methylsulfonyl)-6H-isoquino[2,1-g][1,6]naphthyridi ne, a potent and highly selective alpha 2-adrenoceptor antagonist. J Med Chem. 1989 Sep;32(9):2034–2036. doi: 10.1021/jm00129a002. [DOI] [PubMed] [Google Scholar]
- Clineschmidt B. V., Pettibone D. J., Lotti V. J., Hucker H. B., Sweeney B. M., Reiss D. R., Lis E. V., Huff J. R., Vacca J. A peripherally acting alpha-2 adrenoceptor antagonist: L-659,066. J Pharmacol Exp Ther. 1988 Apr;245(1):32–40. [PubMed] [Google Scholar]
- Convents A., Convents D., De Backer J. P., De Keyser J., Vauquelin G. High affinity binding of 3H rauwolscine and 3H RX781094 to alpha 2 adrenergic receptors and non-stereoselective sites in human and rabbit brain cortex membranes. Biochem Pharmacol. 1989 Feb 1;38(3):455–463. doi: 10.1016/0006-2952(89)90385-7. [DOI] [PubMed] [Google Scholar]
- Goldberg M. R., Hollister A. S., Robertson D. Influence of yohimbine on blood pressure, autonomic reflexes, and plasma catecholamines in humans. Hypertension. 1983 Sep-Oct;5(5):772–778. doi: 10.1161/01.hyp.5.5.772. [DOI] [PubMed] [Google Scholar]
- Goldberg M. R., Robertson D. Yohimbine: a pharmacological probe for study of the alpha 2-adrenoreceptor. Pharmacol Rev. 1983 Sep;35(3):143–180. [PubMed] [Google Scholar]
- Hamilton C. A., Reid J. L., Yakubu M. A. [3H]yohimbine and [3H]idazoxan bind to different sites on rabbit forebrain and kidney membranes. Eur J Pharmacol. 1988 Feb 9;146(2-3):345–348. doi: 10.1016/0014-2999(88)90314-7. [DOI] [PubMed] [Google Scholar]
- Harsing L. G., Vizi E. S. Different sites of action for alpha 2-adrenoceptor antagonists in the modulation of noradrenaline release and contraction response in the vas deferens of the rat. J Pharm Pharmacol. 1992 Mar;44(3):231–234. doi: 10.1111/j.2042-7158.1992.tb03588.x. [DOI] [PubMed] [Google Scholar]
- Hicks P. E., Barras M., Herman G., Mauduit P., Armstrong J. M., Rossignol B. Alpha-adrenoceptor subtypes in dog saphenous vein that mediate contraction and inositol phosphate production. Br J Pharmacol. 1991 Jan;102(1):151–161. doi: 10.1111/j.1476-5381.1991.tb12146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey P. P., Feniuk W., Perren M. J., Connor H. E., Oxford A. W., Coates L. H., Butina D. GR43175, a selective agonist for the 5-HT1-like receptor in dog isolated saphenous vein. Br J Pharmacol. 1988 Aug;94(4):1123–1132. doi: 10.1111/j.1476-5381.1988.tb11630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Limberger N., Trendelenburg A. U., Starke K. Pharmacological characterization of presynaptic alpha 2-autoreceptors in rat submaxillary gland and heart atrium. Br J Pharmacol. 1992 Sep;107(1):246–255. doi: 10.1111/j.1476-5381.1992.tb14494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomasney J. W., Lorenz W., Allen L. F., King K., Regan J. W., Yang-Feng T. L., Caron M. G., Lefkowitz R. J. Expansion of the alpha 2-adrenergic receptor family: cloning and characterization of a human alpha 2-adrenergic receptor subtype, the gene for which is located on chromosome 2. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5094–5098. doi: 10.1073/pnas.87.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald E., Ruskoaho H., Scheinin M., Virtanen R. Therapeutic applications of drugs acting on alpha-adrenoceptors. Ann Clin Res. 1988;20(5):298–310. [PubMed] [Google Scholar]
- MacKinnon A. C., Brown C. M., Spedding M., Kilpatrick A. T. [3H]-idazoxan binds with high affinity to two sites on hamster adipocytes: an alpha 2-adrenoceptor and a non-adrenoceptor site. Br J Pharmacol. 1989 Dec;98(4):1143–1150. doi: 10.1111/j.1476-5381.1989.tb12658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon A. C., Kilpatrick A. T., Kenny B. A., Spedding M., Brown C. M. [3H]-RS-15385-197, a selective and high affinity radioligand for alpha 2-adrenoceptors: implications for receptor classification. Br J Pharmacol. 1992 Aug;106(4):1011–1018. doi: 10.1111/j.1476-5381.1992.tb14449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. C., Brodde O. E., Schnepel B., Behrendt J., Tschada R., Motulsky H. J., Insel P. A. [3H]idazoxan and some other alpha 2-adrenergic drugs also bind with high affinity to a nonadrenergic site. Mol Pharmacol. 1989 Mar;35(3):324–330. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Nichols A. J., Stadel J. M., Hieble J. P. Structure and function of alpha-adrenoceptors. Pharmacol Rev. 1991 Dec;43(4):475–505. [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Zeid R. L. Relationship between alpha adrenoceptor occupancy and response for the alpha-1 adrenoceptor agonist, cirazoline, and the alpha-2 adrenoceptor agonist, B-HT 933, in canine saphenous vein. J Pharmacol Exp Ther. 1985 Dec;235(3):636–643. [PubMed] [Google Scholar]
- Smith K., Connaughton S., Docherty J. R. Investigations of prejunctional alpha 2-adrenoceptors in rat atrium, vas deferens and submandibular gland. Eur J Pharmacol. 1992 Feb 11;211(2):251–256. doi: 10.1016/0014-2999(92)90536-d. [DOI] [PubMed] [Google Scholar]
- Yablonsky F., Riffaud J. P., Lacolle J. Y., Dausse J. P. Evidence for non-adrenergic binding sites for [3H]idazoxan in the smooth muscle of rabbit urethra. Eur J Pharmacol. 1988 Sep 13;154(2):209–212. doi: 10.1016/0014-2999(88)90100-8. [DOI] [PubMed] [Google Scholar]


