Abstract
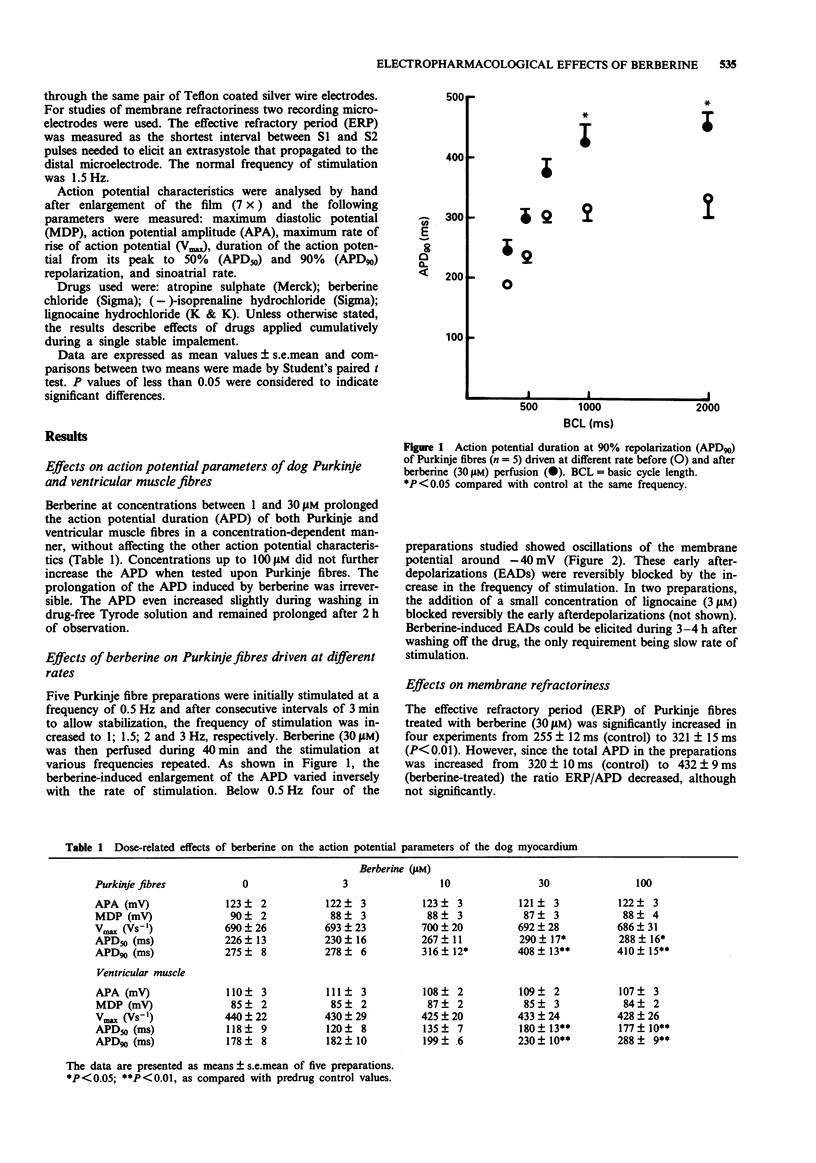
1. Conventional microelectrode techniques were used for intracellular recordings of the transmembrane electrical potentials, the effects of berberine were studied on canine cardiac Purkinje and ventricular muscle fibres and on rabbit atrial fibres. 2. Berberine (3-30 microM) increased in a concentration-dependent manner, the action potential duration (APD) in canine Purkinje and ventricular muscle without affecting other parameters of the action potential. 3. The berberine-induced enlargement of the APD showed reverse use-dependence, so that the effect was greater at lower rates of stimulation. 4. Preparations perfused with berberine (30 microM) and driven at rates below 0.5 Hz exhibited early after depolarizations which persisted 3-4 h after washing. 5. The early afterdepolarizations were reversibly abolished by perfusion with lignocaine (3 microM) or by the increase in the rate of stimulation. 6. The effective refractory period (ERP) of Purkinje fibres was greatly increased by berberine (30 microM); however, the ratio ERP/APD was not significantly affected. 7. Berberine (10-100 microM) decreased in a concentration-dependent manner the spontaneous frequency of rabbit sinoatrial cells. The decrease in frequency was accompanied by a depression of the phase 4 depolarization, without significant changes in other parameters of the nodal action potential. 8. Atropine (2.5 microM) did not affect the bradycardic effect of berberine. On the other hand, berberine (30 microM) did not alter the chronotropic effect of isoprenaline. 9. Berberine (30 microM) also increased the duration of slow responses in K-depolarized rabbit atrial muscle fibres, other parameters being unaffected. 10. It is suggested that berberine exerts Class III antiarrhythmic and proarrhythmic actions in cardiac muscle of the dog in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G. On the relationship between action potential duration and tension in cat papillary muscle. Cardiovasc Res. 1977 May;11(3):210–218. doi: 10.1093/cvr/11.3.210. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Abrahamsson C., Almgren O., Lundberg C., Duker G. Prolonged action potential duration and positive inotropy induced by the novel class III antiarrhythmic agent H 234/09 (Almokalant) in isolated human ventricular muscle. J Cardiovasc Pharmacol. 1991 Dec;18(6):882–887. doi: 10.1097/00005344-199112000-00015. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Almgren O., Duker G. QTU-prolongation and torsades de pointes induced by putative class III antiarrhythmic agents in the rabbit: etiology and interventions. J Cardiovasc Pharmacol. 1990 Aug;16(2):276–285. doi: 10.1097/00005344-199008000-00014. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Electrophysiologic and voltage clamp analysis of the effects of sotalol on isolated cardiac muscle and Purkinje fibers. J Pharmacol Exp Ther. 1985 Mar;232(3):817–825. [PubMed] [Google Scholar]
- Chun Y. T., Yip T. T., Lau K. L., Kong Y. C., Sankawa U. A biochemical study on the hypotensive effect of berberine in rats. Gen Pharmacol. 1979;10(3):177–182. doi: 10.1016/0306-3623(79)90085-5. [DOI] [PubMed] [Google Scholar]
- Davidenko J. M., Cohen L., Goodrow R., Antzelevitch C. Quinidine-induced action potential prolongation, early afterdepolarizations, and triggered activity in canine Purkinje fibers. Effects of stimulation rate, potassium, and magnesium. Circulation. 1989 Mar;79(3):674–686. doi: 10.1161/01.cir.79.3.674. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Snyders D. J. Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation. 1990 Feb;81(2):686–690. doi: 10.1161/01.cir.81.2.686. [DOI] [PubMed] [Google Scholar]
- Huang W. M., Zhang Z. H., Xu Y. Q. Study of the effects and mechanisms of berberine on slow-response action potentials. J Electrocardiol. 1990 Jul;23(3):231–234. doi: 10.1016/0022-0736(90)90161-t. [DOI] [PubMed] [Google Scholar]
- Marin-Neto J. A., Maciel B. C., Secches A. L., Gallo Júnior L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin Cardiol. 1988 Apr;11(4):253–260. doi: 10.1002/clc.4960110411. [DOI] [PubMed] [Google Scholar]
- Reiter M. Calcium mobilization and cardiac inotropic mechanisms. Pharmacol Rev. 1988 Sep;40(3):189–217. [PubMed] [Google Scholar]
- Riccioppo Neto F. Electrophysiological effects of the salicylates on isolated atrial muscle of the rabbit. Br J Pharmacol. 1982 Oct;77(2):285–292. doi: 10.1111/j.1476-5381.1982.tb09297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden D. M., Hoffman B. F. Action potential prolongation and induction of abnormal automaticity by low quinidine concentrations in canine Purkinje fibers. Relationship to potassium and cycle length. Circ Res. 1985 Jun;56(6):857–867. doi: 10.1161/01.res.56.6.857. [DOI] [PubMed] [Google Scholar]
- Rozanski G. J., Witt R. C. Early afterdepolarizations and triggered activity in rabbit cardiac Purkinje fibers recovering from ischemic-like conditions. Role of acidosis. Circulation. 1991 Apr;83(4):1352–1360. doi: 10.1161/01.cir.83.4.1352. [DOI] [PubMed] [Google Scholar]
- Sabir M., Akhter M. H., Bhide N. K. Further studies on pharmacology of berberine. Indian J Physiol Pharmacol. 1978 Jan-Mar;22(1):9–23. [PubMed] [Google Scholar]
- Sabir M., Bhide N. K. Study of some pharmacological actions of berberine. Indian J Physiol Pharmacol. 1971 Jul;15(3):111–132. [PubMed] [Google Scholar]
- Satoh H. Class III antiarrhythmic drugs (amiodarone, bretylium and sotalol) on action potentials and membrane currents in rabbit sino-atrial node preparations. Naunyn Schmiedebergs Arch Pharmacol. 1991 Dec;344(6):674–681. doi: 10.1007/BF00174751. [DOI] [PubMed] [Google Scholar]
- Shaffer J. E. Inotropic and chronotropic activity of berberine on isolated guinea pig atria. J Cardiovasc Pharmacol. 1985 Mar-Apr;7(2):307–315. doi: 10.1097/00005344-198503000-00016. [DOI] [PubMed] [Google Scholar]
- Strauss H. C., Bigger J. T., Jr, Hoffman B. F. Electrophysiologial and beta-receptor blocking effects of MJ 1999 on dog and rabbit cardiac tissue. Circ Res. 1970 Jun;26(6):661–678. doi: 10.1161/01.res.26.6.661. [DOI] [PubMed] [Google Scholar]
- Vaughan Williams E. M. A classification of antiarrhythmic actions reassessed after a decade of new drugs. J Clin Pharmacol. 1984 Apr;24(4):129–147. doi: 10.1002/j.1552-4604.1984.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Harsing L. G., Jr, Gaal J., Kapocsi J., Bernath S., Somogyi G. T. CH-38083, a selective, potent antagonist of alpha-2 adrenoceptors. J Pharmacol Exp Ther. 1986 Aug;238(2):701–706. [PubMed] [Google Scholar]
- el-Sherif N., Bekheit S. S., Henkin R. Quinidine-induced long QTU interval and torsade de pointes: role of bradycardia-dependent early afterdepolarizations. J Am Coll Cardiol. 1989 Jul;14(1):252–257. doi: 10.1016/0735-1097(89)90082-x. [DOI] [PubMed] [Google Scholar]



