Abstract
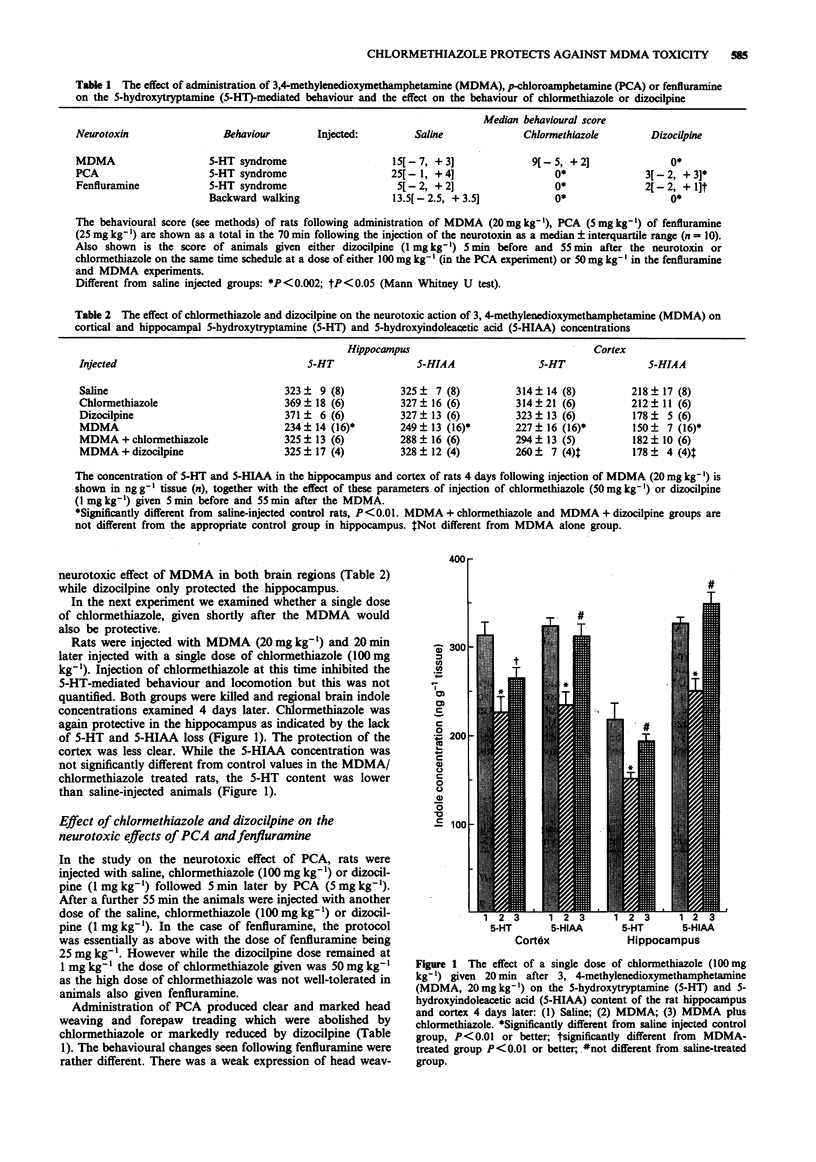
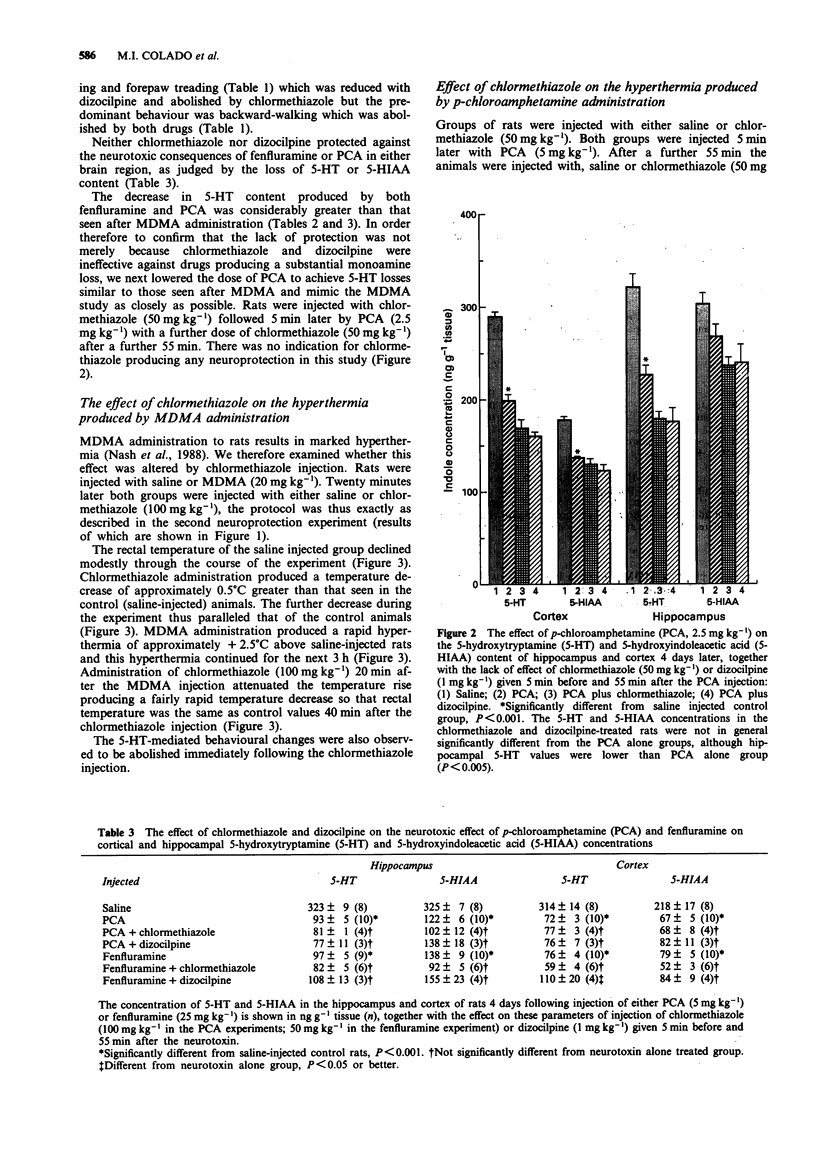
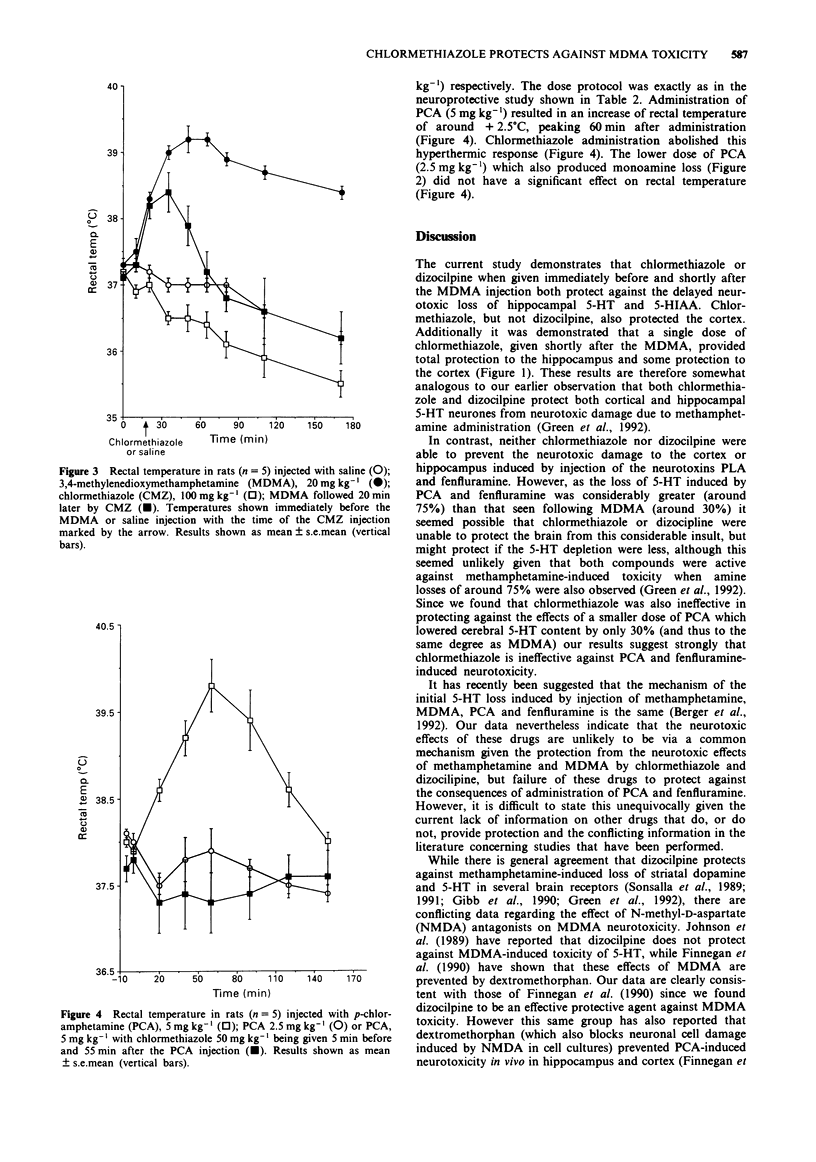
1. The present study has investigated whether the neurotoxic effects of the relatively selective 5-hydroxytryptamine (5-HT) neurotoxins, 3,4-methylenedioxymethamphetamine (MDMA or 'Ecstasy'), p-chloroamphetamine (PCA) and fenfluramine on hippocampal and cortical 5-HT terminals in rat brain could be prevented by administration of either chlormethiazole or dizocilpine. 2. Administration of MDMA (20 mg kg-1, i.p.) resulted in an approximate 30% loss of cortical and hippocampal 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) content 4 days later. Injection of chlormethiazole (50 mg kg-1) 5 min before and 55 min after the MDMA provided complete protection in both regions, while dizocilpine (1 mg kg-1, i.p.) protected only the hippocampus. 3. Administration of a single dose of chlormethiazole (100 mg kg-1) 20 min after the MDMA also provided complete protection to the hippocampus but not the cortex. This regime also attenuated the sustained hyperthermia (approx +2.5 degrees C) induced by the MDMA injection. 4. Injection of PCA (5 mg kg-1, i.p.) resulted in a 70% loss of 5-HT and 5-HIAA content in hippocampus and cortex 4 days later. Injection of chlormethiazole (100 mg kg-1, i.p.) or dizocilpine (1 mg kg-1, i.p.) 5 min before and 55 min after the PCA failed to protect against the neurotoxicity, nor was protection afforded by chlormethiazole when a lower dose of PCA (2.5 mg kg-1, i.p.) was given which produced only a 30% loss of 5-HT content.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger U. V., Gu X. F., Azmitia E. C. The substituted amphetamines 3,4-methylenedioxymethamphetamine, methamphetamine, p-chloroamphetamine and fenfluramine induce 5-hydroxytryptamine release via a common mechanism blocked by fluoxetine and cocaine. Eur J Pharmacol. 1992 May 14;215(2-3):153–160. doi: 10.1016/0014-2999(92)90023-w. [DOI] [PubMed] [Google Scholar]
- Cross A. J., Jones J. A., Baldwin H. A., Green A. R. Neuroprotective activity of chlormethiazole following transient forebrain ischaemia in the gerbil. Br J Pharmacol. 1991 Oct;104(2):406–411. doi: 10.1111/j.1476-5381.1991.tb12443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. J., Stirling J. M., Robinson T. N., Bowen D. M., Francis P. T., Green A. R. The modulation by chlormethiazole of the GABAA-receptor complex in rat brain. Br J Pharmacol. 1989 Sep;98(1):284–290. doi: 10.1111/j.1476-5381.1989.tb16893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G., Fernando J. C., Lees A. J. Backward walking and circling: behavioural responses induced by drug treatments which cause simultaneous release of catecholamines and 5-hydroxytryptamine. Br J Pharmacol. 1979 Aug;66(4):573–579. doi: 10.1111/j.1476-5381.1979.tb13696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin J. F., Green A. R. The effects of putative 5-hydroxytryptamine antagonists on the behaviour produced by administration of tranylcypromine and L-tryptophan or tranylcypromine and L-DOPA to rats. Br J Pharmacol. 1978 Oct;64(2):201–209. doi: 10.1111/j.1476-5381.1978.tb17290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan K. T., Kerr J. T., Langston J. W. Dextromethorphan protects against the neurotoxic effects of p-chloroamphetamine in rats. Brain Res. 1991 Aug 30;558(1):109–111. doi: 10.1016/0006-8993(91)90723-9. [DOI] [PubMed] [Google Scholar]
- Finnegan K. T., Skratt J. J., Irwin I., Langston J. W. The N-methyl-D-aspartate (NMDA) receptor antagonist, dextrorphan, prevents the neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neurosci Lett. 1989 Nov 6;105(3):300–306. doi: 10.1016/0304-3940(89)90637-x. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Perry K. W., Molloy B. B. Reversible and irreversible phases of serotonin depletion by 4-chloroamphetamine. Eur J Pharmacol. 1975 Aug;33(1):119–124. doi: 10.1016/0014-2999(75)90145-4. [DOI] [PubMed] [Google Scholar]
- Gibb J. W., Johnson M., Stone D., Hanson G. R. MDMA: historical perspectives. Ann N Y Acad Sci. 1990;600:601–612. doi: 10.1111/j.1749-6632.1990.tb16913.x. [DOI] [PubMed] [Google Scholar]
- Gill R., Foster A. C., Woodruff G. N. MK-801 is neuroprotective in gerbils when administered during the post-ischaemic period. Neuroscience. 1988 Jun;25(3):847–855. doi: 10.1016/0306-4522(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Gill R., Foster A. C., Woodruff G. N. Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. J Neurosci. 1987 Oct;7(10):3343–3349. doi: 10.1523/JNEUROSCI.07-10-03343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., De Souza R. J., Williams J. L., Murray T. K., Cross A. J. The neurotoxic effects of methamphetamine on 5-hydroxytryptamine and dopamine in brain: evidence for the protective effect of chlormethiazole. Neuropharmacology. 1992 Apr;31(4):315–321. doi: 10.1016/0028-3908(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Green A. R., Kelly P. H. Evidence concerning the involvement of 5-hydroxytryptamine in the locomotor activity produced by amphetamine or tranylcypromine plus L-DOPA. Br J Pharmacol. 1976 May;57(1):141–147. doi: 10.1111/j.1476-5381.1976.tb07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Murray T. K. A simple intravenous infusion method in rodents for determining the potency of anticonvulsants acting through GABAergic mechanisms. J Pharm Pharmacol. 1989 Dec;41(12):879–880. doi: 10.1111/j.2042-7158.1989.tb06395.x. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Two distinct interactions of barbiturates and chlormethiazole with the GABAA receptor complex in rat cuneate nucleus in vitro. Br J Pharmacol. 1983 Oct;80(2):387–394. doi: 10.1111/j.1476-5381.1983.tb10045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. A., McMaster S. E. Fenfluramine: evidence for a neurotoxic action on midbrain and a long-term depletion of serotonin. Psychopharmacol Commun. 1975;1(2):217–228. [PubMed] [Google Scholar]
- Hemrick-Luecke S. K., Henderson M. G., Fuller R. W. MK801 antagonism of the prolonged depletion of striatal dopamine by amphetamine in iprindole-treated rats. Life Sci. 1992;50(6):PL31–PL33. doi: 10.1016/0024-3205(92)90383-z. [DOI] [PubMed] [Google Scholar]
- Hotchkiss A. J., Gibb J. W. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980 Aug;214(2):257–262. [PubMed] [Google Scholar]
- Johnson M. P., Huang X. M., Nichols D. E. Serotonin neurotoxicity in rats after combined treatment with a dopaminergic agent followed by a nonneurotoxic 3,4-methylenedioxymethamphetamine (MDMA) analogue. Pharmacol Biochem Behav. 1991 Dec;40(4):915–922. doi: 10.1016/0091-3057(91)90106-c. [DOI] [PubMed] [Google Scholar]
- Johnson M., Hanson G. R., Gibb J. W. Effect of MK-801 on the decrease in tryptophan hydroxylase induced by methamphetamine and its methylenedioxy analog. Eur J Pharmacol. 1989 Jun 20;165(2-3):315–318. doi: 10.1016/0014-2999(89)90728-0. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Nwanze E. Selective (+)-amphetamine neurotoxicity on striatal dopamine nerve terminals in the mouse. Br J Pharmacol. 1982 Oct;77(2):335–345. doi: 10.1111/j.1476-5381.1982.tb09303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda L. Y., Gibb J. W. Adrenal and striatal tyrosine hydroxylase activity after methamphetamine. J Pharmacol Exp Ther. 1973 Apr;185(1):42–48. [PubMed] [Google Scholar]
- McKenna D. J., Peroutka S. J. Neurochemistry and neurotoxicity of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy"). J Neurochem. 1990 Jan;54(1):14–22. doi: 10.1111/j.1471-4159.1990.tb13277.x. [DOI] [PubMed] [Google Scholar]
- Nash J. F., Jr, Meltzer H. Y., Gudelsky G. A. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988 Jun;245(3):873–879. [PubMed] [Google Scholar]
- Ricaurte G. A., Guillery R. W., Seiden L. S., Schuster C. R., Moore R. Y. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982 Mar 4;235(1):93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E., Bushing J. A., Sulser F. Long-term effects of p-chloroamphetamine on tryptophan hydroxylase activity and on the levels of 5-hydroxytryptamine and 5-hydroxyindole acetic acid in brain. Eur J Pharmacol. 1972 Dec;20(3):385–388. doi: 10.1016/0014-2999(72)90204-x. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Black C. K., Taylor V. L. L-DOPA potentiation of the serotonergic deficits due to a single administration of 3,4-methylenedioxymethamphetamine, p-chloroamphetamine or methamphetamine to rats. Eur J Pharmacol. 1991 Oct 2;203(1):41–49. doi: 10.1016/0014-2999(91)90788-r. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Kehne J. H. Neurotoxicity of MDMA: neurochemical effects. Ann N Y Acad Sci. 1990;600:665–681. doi: 10.1111/j.1749-6632.1990.tb16917.x. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1987 Jan;240(1):1–7. [PubMed] [Google Scholar]
- Schmidt C. J., Ritter J. K., Sonsalla P. K., Hanson G. R., Gibb J. W. Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 1985 Jun;233(3):539–544. [PubMed] [Google Scholar]
- Sonsalla P. K., Nicklas W. J., Heikkila R. E. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989 Jan 20;243(4889):398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- Sonsalla P. K., Riordan D. E., Heikkila R. E. Competitive and noncompetitive antagonists at N-methyl-D-aspartate receptors protect against methamphetamine-induced dopaminergic damage in mice. J Pharmacol Exp Ther. 1991 Feb;256(2):506–512. [PubMed] [Google Scholar]
- Stone D. M., Johnson M., Hanson G. R., Gibb J. W. Role of endogenous dopamine in the central serotonergic deficits induced by 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988 Oct;247(1):79–87. [PubMed] [Google Scholar]
- Stone D. M., Stahl D. C., Hanson G. R., Gibb J. W. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur J Pharmacol. 1986 Aug 22;128(1-2):41–48. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- Tricklebank M. D., Singh L., Oles R. J., Preston C., Iversen S. D. The behavioural effects of MK-801: a comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur J Pharmacol. 1989 Aug 11;167(1):127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]


