Abstract
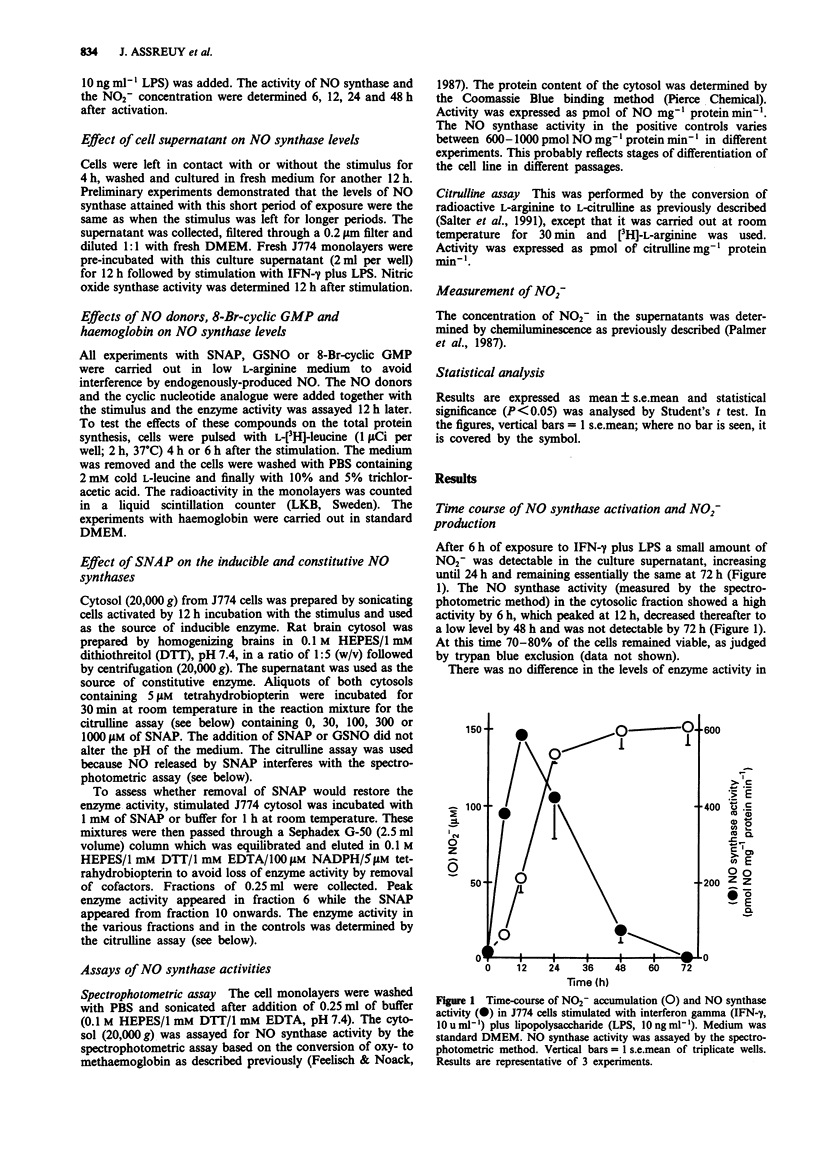
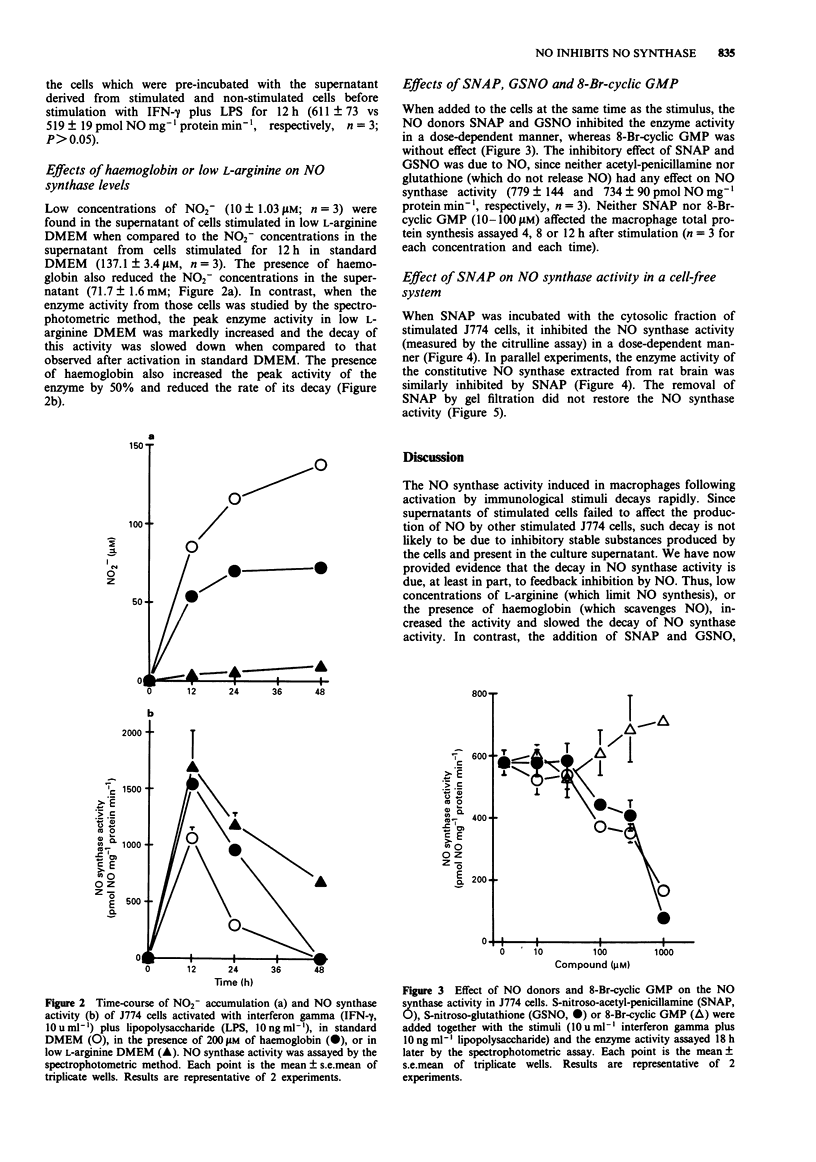
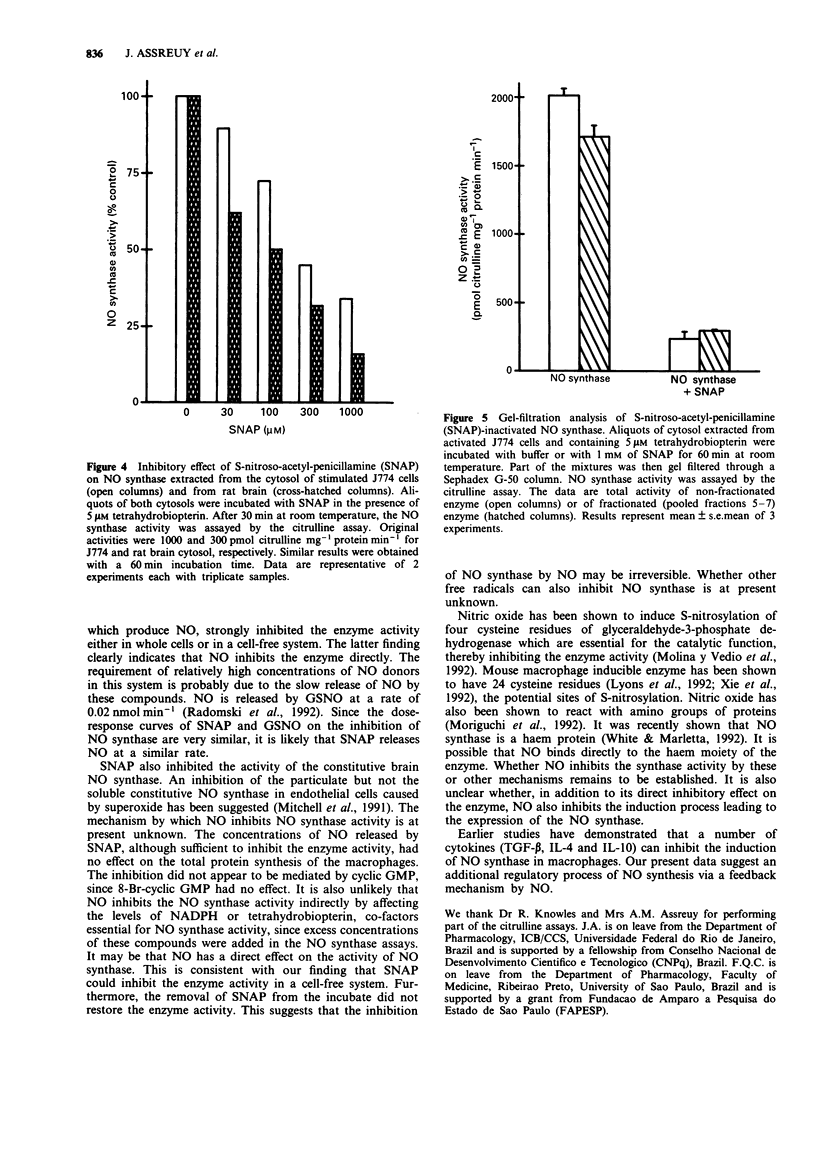
1. A murine macrophage cell line, J774, expressed nitric oxide (NO) synthase activity in response to interferon-gamma (IFN-gamma, 10 u ml-1) plus lipopolysaccharide (LPS, 10 ng ml-1). The enzyme activity was first detectable 6 h after incubation, peaked at 12 h and became undetectable after 48 h. 2. The decline in the NO synthase activity was not due to inhibition by stable substances secreted by the cells into the culture supernatant. 3. The decline in the NO synthase activity was significantly slowed down in cells cultured in a low L-arginine medium or with added haemoglobin, suggesting that NO may be involved in a feedback inhibitory mechanism. 4. The addition of NO generators, S-nitroso-acetyl-penicillamine (SNAP) or S-nitroso-glutathione (GSNO) markedly inhibited the NO synthase activity in a dose-dependent manner. The effect of NO on the enzyme was not due to the inhibition of de novo protein synthesis. 5. SNAP directly inhibited the inducible NO synthase extracted from activated J774 cells, as well as the constitutive NO synthase extracted from the rat brain. 6. The enzyme activity of J774 cells was not restored after the removal of SNAP by gel filtration, suggesting that NO inhibits NO synthase irreversibly.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunha F. Q., Moncada S., Liew F. Y. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1155–1159. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Guild S. Effects of adenosine 3':5'-cyclic monophosphate and guanine nucleotides on calcium-evoked ACTH release from electrically permeabilized AtT-20 cells. Br J Pharmacol. 1991 Sep;104(1):117–122. doi: 10.1111/j.1476-5381.1991.tb12394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Severn A., Millott S., Schmidt J., Salter M., Moncada S. A possible novel pathway of regulation by murine T helper type-2 (Th2) cells of a Th1 cell activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur J Immunol. 1991 Oct;21(10):2489–2494. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moriguchi M., Manning L. R., Manning J. M. Nitric oxide can modify amino acid residues in proteins. Biochem Biophys Res Commun. 1992 Mar 16;183(2):598–604. doi: 10.1016/0006-291x(92)90524-o. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Rees D. D., Dutra A., Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992 Nov;107(3):745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Takema M., Inaba K., Uno K., Kakihara K., Tawara K., Muramatsu S. Effect of L-arginine on the retention of macrophage tumoricidal activity. J Immunol. 1991 Mar 15;146(6):1928–1933. [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]


