Abstract
1. The ability of capsazepine, a recently developed capsaicin receptor antagonist, to prevent the effects of capsaicin on the rat isolated urinary bladder (contraction) and vas deferens (inhibition of electrically-evoked twitches) was compared to that of ruthenium red, a dye which behaves as a functional antagonist of capsaicin. 2. In the rat bladder, capsazepine (3-30 microM) produced a concentration-dependent rightward shift of the curve to capsaicin without any significant depression of the maximal response to the agonist. By contrast, ruthenium red (10-30 microM) produced a non-competitive type of antagonism, characterized by marked depression of the maximal response attainable. Similar findings were obtained in the rat isolated vas deferens in which capsazepine (10 microM) produced a rightward shift of the curve to capsaicin while ruthenium red (3 microM) depressed the maximal response to the agonist. 3. At the concentrations used to block the effect of capsaicin, neither capsazepine nor ruthenium red affected the contractile response of the rat urinary bladder produced by either neurokinin A or electrical field stimulation or the twitch inhibition produced by rat alpha-calcitonin gene-related peptide (alpha CGRP) in the vas deferens. 4. These findings provide additional evidence that both capsazepine and ruthenium red are valuable tools for exploration of the function of capsaicin-sensitive primary afferent neurones. The antagonism of the action of capsaicin by capsazepine is entirely consistent with the proposed interaction of this substance with a vanilloid receptor located on primary afferents, while the action of ruthenium red apparently involves a more complex, non-competitive antagonism.
Full text
PDF
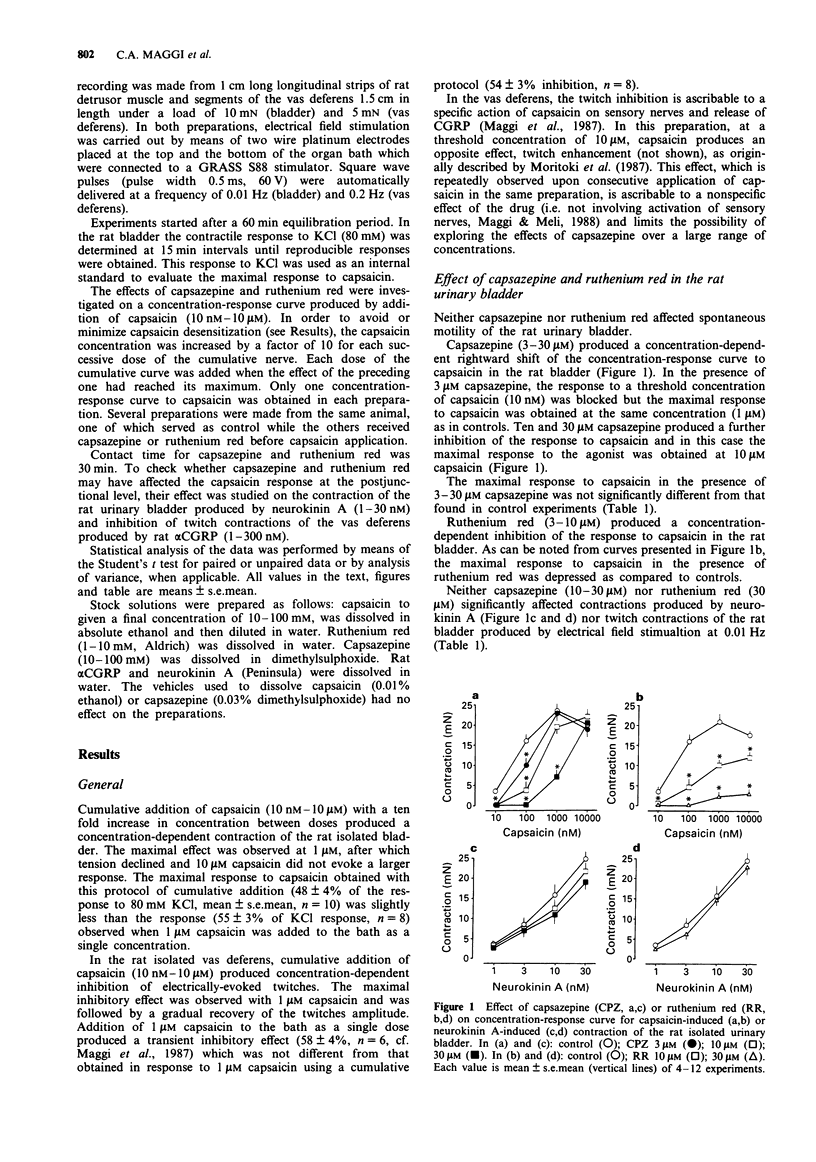
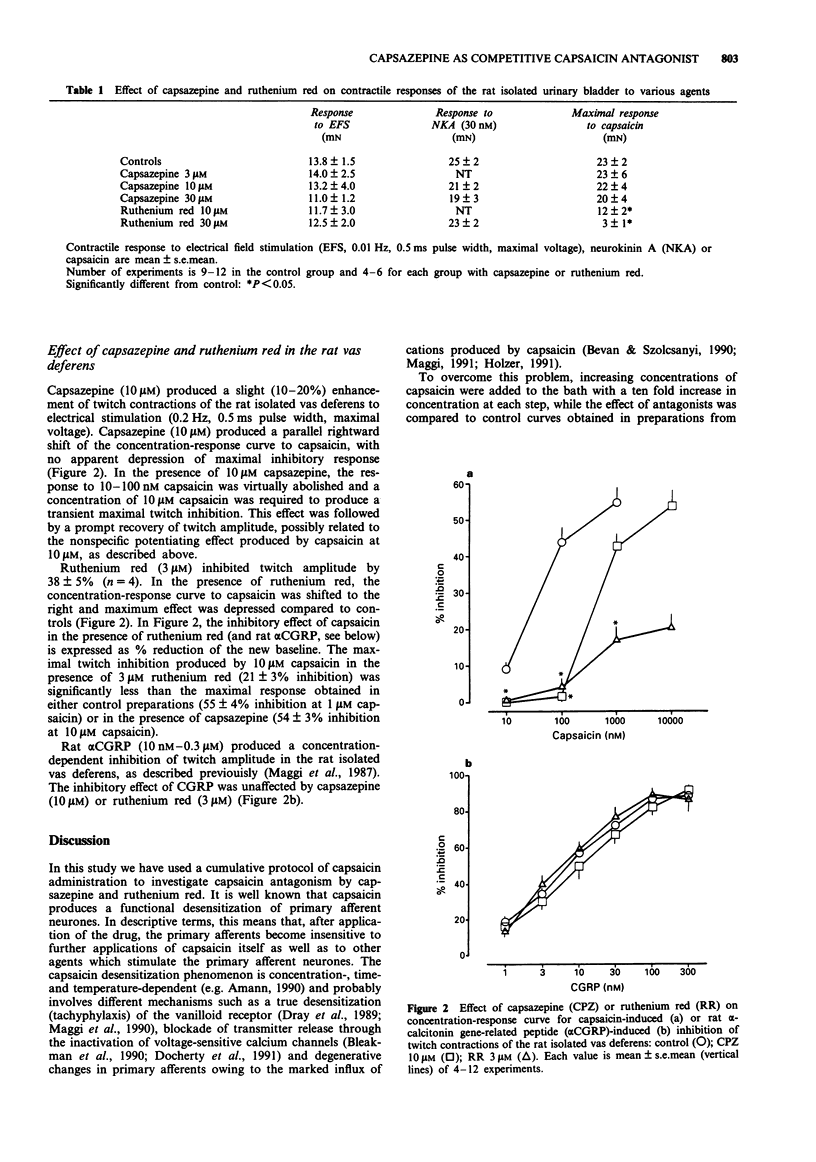


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. Desensitization of capsaicin-evoked neuropeptide release--influence of Ca2+ and temperature. Naunyn Schmiedebergs Arch Pharmacol. 1990 Dec;342(6):671–676. doi: 10.1007/BF00175711. [DOI] [PubMed] [Google Scholar]
- Amann R., Maggi C. A. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49(12):849–856. doi: 10.1016/0024-3205(91)90169-c. [DOI] [PubMed] [Google Scholar]
- Bevan S., Hothi S., Hughes G., James I. F., Rang H. P., Shah K., Walpole C. S., Yeats J. C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992 Oct;107(2):544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Szolcsányi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci. 1990 Aug;11(8):330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- Bleakman D., Brorson J. R., Miller R. J. The effect of capsaicin on voltage-gated calcium currents and calcium signals in cultured dorsal root ganglion cells. Br J Pharmacol. 1990 Oct;101(2):423–431. doi: 10.1111/j.1476-5381.1990.tb12725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahl L. A. The effects of ruthenium red on the response of guinea-pig ileum to capsaicin. Eur J Pharmacol. 1989 Oct 10;169(2-3):241–247. doi: 10.1016/0014-2999(89)90021-6. [DOI] [PubMed] [Google Scholar]
- Docherty R. J., Robertson B., Bevan S. Capsaicin causes prolonged inhibition of voltage-activated calcium currents in adult rat dorsal root ganglion neurons in culture. Neuroscience. 1991;40(2):513–521. doi: 10.1016/0306-4522(91)90137-d. [DOI] [PubMed] [Google Scholar]
- Dray A., Bettaney J., Forster P. Capsaicin desensitization of peripheral nociceptive fibres does not impair sensitivity to other noxious stimuli. Neurosci Lett. 1989 Apr 24;99(1-2):50–54. doi: 10.1016/0304-3940(89)90263-2. [DOI] [PubMed] [Google Scholar]
- Dray A., Forbes C. A., Burgess G. M. Ruthenium red blocks the capsaicin-induced increase in intracellular calcium and activation of membrane currents in sensory neurones as well as the activation of peripheral nociceptors in vitro. Neurosci Lett. 1990 Mar 2;110(1-2):52–59. doi: 10.1016/0304-3940(90)90786-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143–201. [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Astolfi M., Donnerer J., Amann R. Which mechanisms account for the sensory neuron blocking action of capsaicin on primary afferents in the rat urinary bladder? Neurosci Lett. 1990 Mar 14;110(3):267–272. doi: 10.1016/0304-3940(90)90858-7. [DOI] [PubMed] [Google Scholar]
- Maggi C. A. Capsaicin and primary afferent neurons: from basic science to human therapy? J Auton Nerv Syst. 1991 Apr;33(1):1–14. doi: 10.1016/0165-1838(91)90013-s. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Santicioli P., Meli A. Capsaicin-induced inhibition of motility of the rat isolated vas deferens: do multiple neuropeptides mediate the visceromotor effects of capsaicin? J Auton Pharmacol. 1987 Sep;7(3):243–255. doi: 10.1111/j.1474-8673.1987.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Santicioli P., Giuliani S., Geppetti P., Meli A. Protective action of ruthenium red toward capsaicin desensitization of sensory fibers. Neurosci Lett. 1988 May 26;88(2):201–205. doi: 10.1016/0304-3940(88)90126-7. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Santicioli P., Giuliani S. Tachykinin antagonists and capsaicin-induced contraction of the rat isolated urinary bladder: evidence for tachykinin-mediated cotransmission. Br J Pharmacol. 1991 Jun;103(2):1535–1541. doi: 10.1111/j.1476-5381.1991.tb09823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Geppetti P., Parlani M., Astolfi M., Pradelles P., Patacchini R., Meli A. The antagonism induced by ruthenium red of the actions of capsaicin on the peripheral terminals of sensory neurons: further studies. Eur J Pharmacol. 1988 Sep 1;154(1):1–10. doi: 10.1016/0014-2999(88)90356-1. [DOI] [PubMed] [Google Scholar]
- Moritoki H., Iwamoto T., Kanaya J., Ishida Y., Ando K., Kitagawa K. Capsaicin enhances the non-adrenergic twitch response of rat vas deferens. Br J Pharmacol. 1987 Oct;92(2):469–475. doi: 10.1111/j.1476-5381.1987.tb11344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santicioli P., Maggi C. A., Geppetti P., Del Bianco E., Theodorsson E., Meli A. Release of calcitonin gene-related peptide-like immunoreactivity (CGRP-LI) from organs of the genitourinary tract in rats. Neurosci Lett. 1988 Oct 5;92(2):197–201. doi: 10.1016/0304-3940(88)90060-2. [DOI] [PubMed] [Google Scholar]
- Szallasi A., Blumberg P. M. Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root ganglion membranes. Brain Res. 1990 Jul 30;524(1):106–111. doi: 10.1016/0006-8993(90)90498-z. [DOI] [PubMed] [Google Scholar]
- Urban L., Dray A. Capsazepine, a novel capsaicin antagonist, selectively antagonises the effects of capsaicin in the mouse spinal cord in vitro. Neurosci Lett. 1991 Dec 16;134(1):9–11. doi: 10.1016/0304-3940(91)90496-g. [DOI] [PubMed] [Google Scholar]
- Webber S. E., Lim J. C., Widdicombe J. G. The effects of calcitonin gene-related peptide on submucosal gland secretion and epithelial albumin transport in the ferret trachea in vitro. Br J Pharmacol. 1991 Jan;102(1):79–84. doi: 10.1111/j.1476-5381.1991.tb12135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


