Abstract
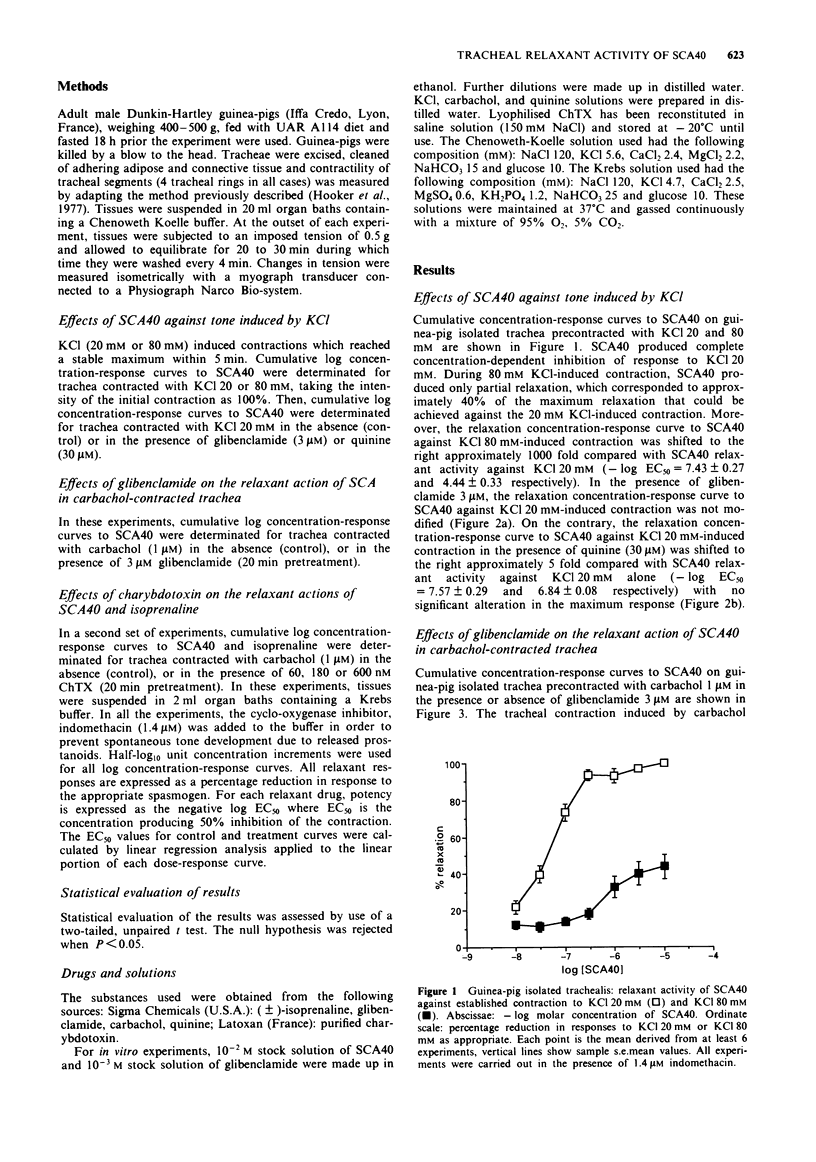
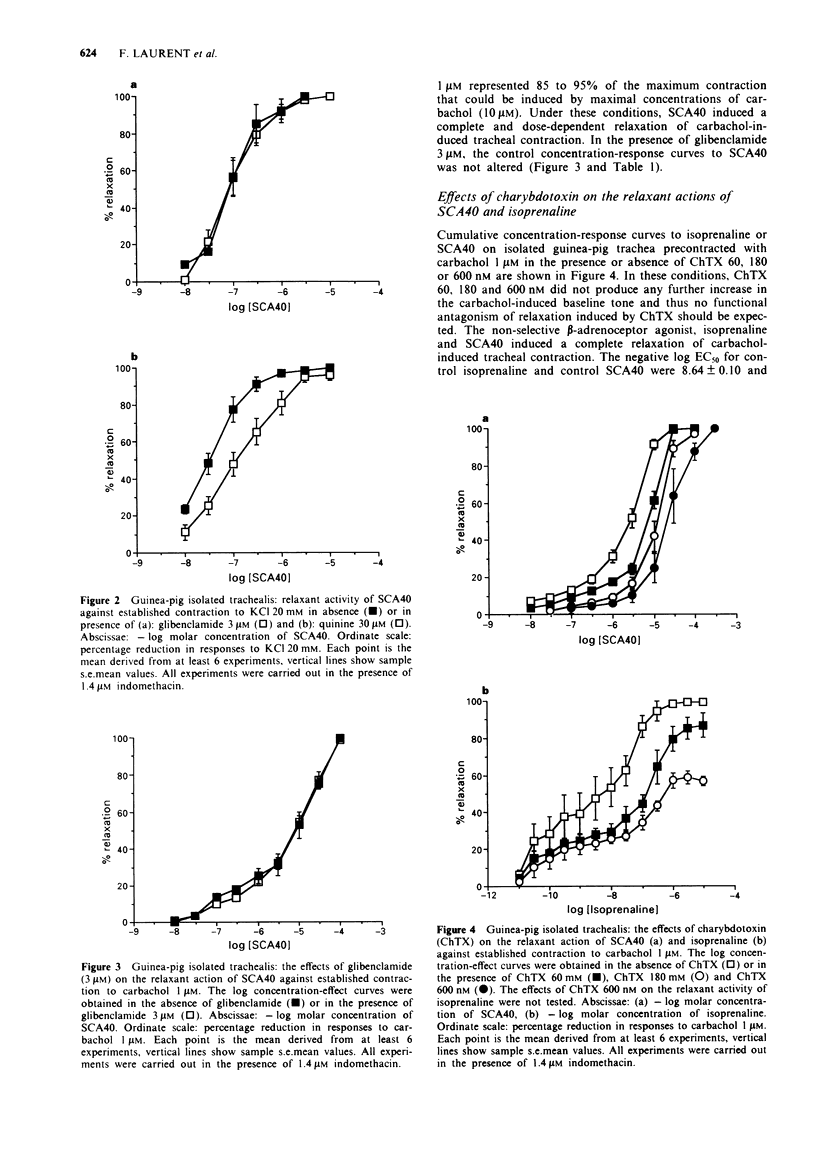
1. Experiments have been performed in order to analyse the mechanism whereby SCA40, a new imidazo[1,2-a]pyrazine derivative relaxes airway smooth muscle. 2. SCA40 (0.01-10 microM) caused a complete and concentration-dependent relaxation of guinea-pig isolated trachea contracted with 20 mM KCl but failed to inhibit completely the spasmogenic effects of 80 mM KCl. 3. Quinine (30 microM) antagonized the relaxant activity of SCA40 in 20 mM KCl-contracted guinea-pig isolated trachea. The ATP-sensitive K(+)-channel blocker, glibenclamide (3 microM), did not antagonize the relaxant activity of SCA40 in either 20 mM KCl or 1 microM carbachol-contracted isolated trachea. 4. SCA40 (0.01-10 microM) and isoprenaline (0.1 nM-10 microM) caused a complete and concentration-dependent relaxation of guinea-pig isolated trachea contracted with carbachol 1 microM. 5. The large-conductance Ca(2+)-activated K(+)-channel blocker, charybdotoxin (60-180 nM), non-competitively antagonized the relaxant activity of isoprenaline on 1 microM carbachol-contracted trachea. The inhibition was characterized by rightward shifts of the isoprenaline concentration-relaxation curves with depression of their maxima. 6. The relaxant activity of SCA40 in 1 microM carbachol-contracted trachea was antagonized by charybdotoxin (60-600 nM) in an apparently competitive manner. The concentration-relaxation curves to SCA40 were shifted to the right with no significant alteration in the maximum response. 7. It is concluded that SCA40 is a novel potassium channel opener which is a potent relaxant of guinea-pig airway smooth muscle in vitro.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Beech D. J., Foster R. W., Morgan G. P., Small R. C. Electrophysiological and other aspects of the relaxant action of isoprenaline in guinea-pig isolated trachealis. Br J Pharmacol. 1985 Dec;86(4):843–854. doi: 10.1111/j.1476-5381.1985.tb11106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. L., Boyle J. P., Cortijo J., Foster R. W., Morgan G. P., Small R. C. Electrical and mechanical effects of BRL34915 in guinea-pig isolated trachealis. Br J Pharmacol. 1986 Oct;89(2):395–405. doi: 10.1111/j.1476-5381.1986.tb10273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B., Romey G., Honoré E., Schmid-Alliana A., Mattéi M. G., Lesage F., Ricard P., Barhanin J., Lazdunski M. Cloning, functional expression, and regulation of two K+ channels in human T lymphocytes. J Biol Chem. 1992 Apr 25;267(12):8650–8657. [PubMed] [Google Scholar]
- Bonnet P. A., Michel A., Laurent F., Sablayrolles C., Rechencq E., Mani J. C., Boucard M., Chapat J. P. Synthesis and antibronchospastic activity of 8-alkoxy- and 8-(alkylamino)imidazo[1,2-a]pyrazines. J Med Chem. 1992 Sep 4;35(18):3353–3358. doi: 10.1021/jm00096a008. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G., Jenkinson D. H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989 Feb;12(2):59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Navia M. A., Reuben J. P., Katz G. M., Kaczorowski G. J., Garcia M. L. Purification, sequence, and model structure of charybdotoxin, a potent selective inhibitor of calcium-activated potassium channels. Proc Natl Acad Sci U S A. 1988 May;85(10):3329–3333. doi: 10.1073/pnas.85.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. A., Foster R. W., Small R. C. A patch-clamp study of K(+)-channel activity in bovine isolated tracheal smooth muscle cells. Br J Pharmacol. 1991 Apr;102(4):871–878. doi: 10.1111/j.1476-5381.1991.tb12269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C. S., Calkins P. J., Fleisch J. H. On the measurement of vascular and respiratory smooth muscle responses in vitro. Blood Vessels. 1977;14(1):1–11. doi: 10.1159/000158110. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Charette L., Garcia M. L., Kaczorowski G. J. Selective inhibition of relaxation of guinea-pig trachea by charybdotoxin, a potent Ca(++)-activated K+ channel inhibitor. J Pharmacol Exp Ther. 1990 Nov;255(2):697–706. [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellemkjaer S., Nielsen-Kudsk J. E., Nielsen C. B., Siggaard C. A comparison of the relaxant effects of pinacidil in guinea-pig trachea, aorta and pulmonary artery. Eur J Pharmacol. 1989 Aug 22;167(2):275–280. doi: 10.1016/0014-2999(89)90588-8. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Berry J. L., Cook S. J., Foster R. W., Green K. A., Small R. C. Guinea-pig isolated trachealis: the effects of charybdotoxin on mechanical activity, membrane potential changes and the activity of plasmalemmal K(+)-channels. Br J Pharmacol. 1991 Jul;103(3):1814–1818. doi: 10.1111/j.1476-5381.1991.tb09868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast U., Cook N. S. Moving together: K+ channel openers and ATP-sensitive K+ channels. Trends Pharmacol Sci. 1989 Nov;10(11):431–435. doi: 10.1016/S0165-6147(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Robertson D. W., Steinberg M. I. Potassium channel modulators: scientific applications and therapeutic promise. J Med Chem. 1990 Jun;33(6):1529–1541. doi: 10.1021/jm00168a001. [DOI] [PubMed] [Google Scholar]
- Sablayrolles C., Cros G. H., Milhavet J. C., Rechenq E., Chapat J. P., Boucard M., Serrano J. J., McNeill J. H. Synthesis of imidazo[1,2-a]pyrazine derivatives with uterine-relaxing, antibronchospastic, and cardiac-stimulating properties. J Med Chem. 1984 Feb;27(2):206–212. doi: 10.1021/jm00368a018. [DOI] [PubMed] [Google Scholar]
- Small R. C., Boyle J. P., Duty S., Elliott K. R., Foster R. W., Watt A. J. Analysis of the relaxant effects of AH 21-132 in guinea-pig isolated trachealis. Br J Pharmacol. 1989 Aug;97(4):1165–1173. doi: 10.1111/j.1476-5381.1989.tb12575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Renterghem C., Lazdunski M. A small-conductance charybdotoxin-sensitive, apamin-resistant Ca(2+)-activated K+ channel in aortic smooth muscle cells (A7r5 line and primary culture). Pflugers Arch. 1992 Apr;420(5-6):417–423. doi: 10.1007/BF00374614. [DOI] [PubMed] [Google Scholar]
- Vázquez J., Feigenbaum P., Katz G., King V. F., Reuben J. P., Roy-Contancin L., Slaughter R. S., Kaczorowski G. J., Garcia M. L. Characterization of high affinity binding sites for charybdotoxin in sarcolemmal membranes from bovine aortic smooth muscle. Evidence for a direct association with the high conductance calcium-activated potassium channel. J Biol Chem. 1989 Dec 15;264(35):20902–20909. [PubMed] [Google Scholar]


