Abstract
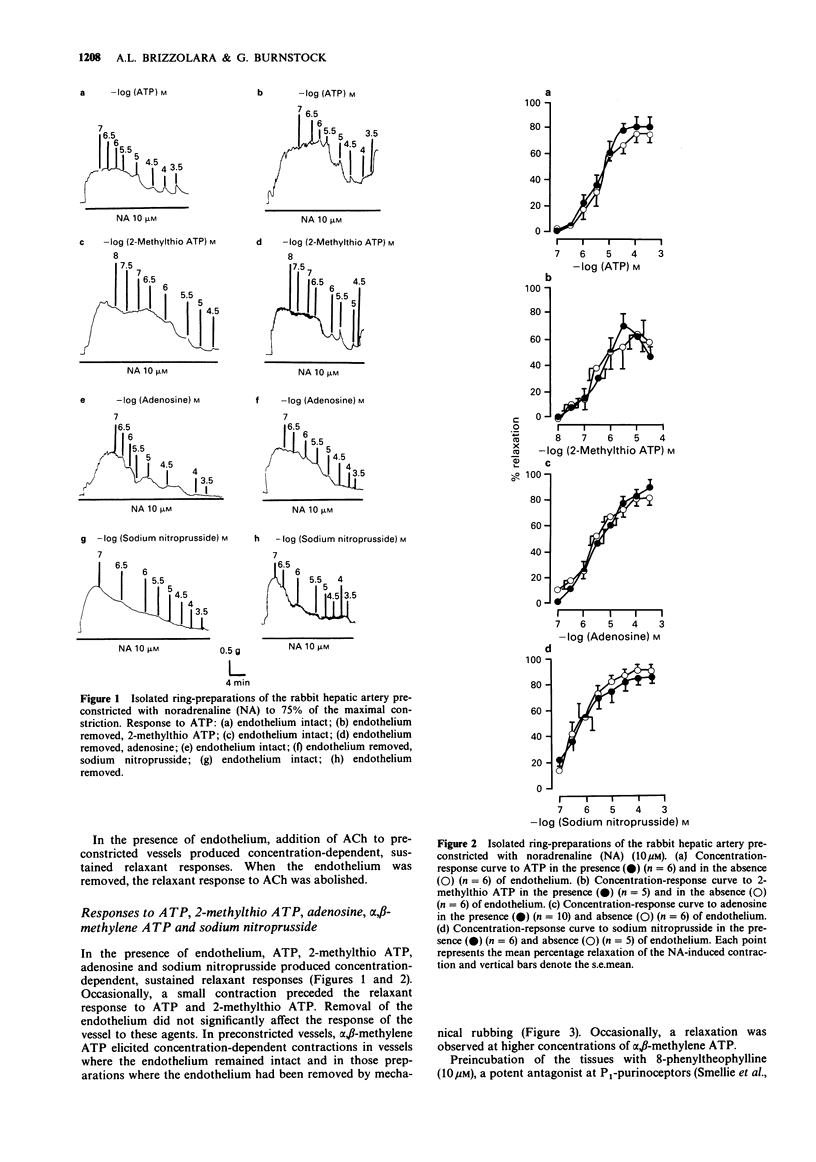
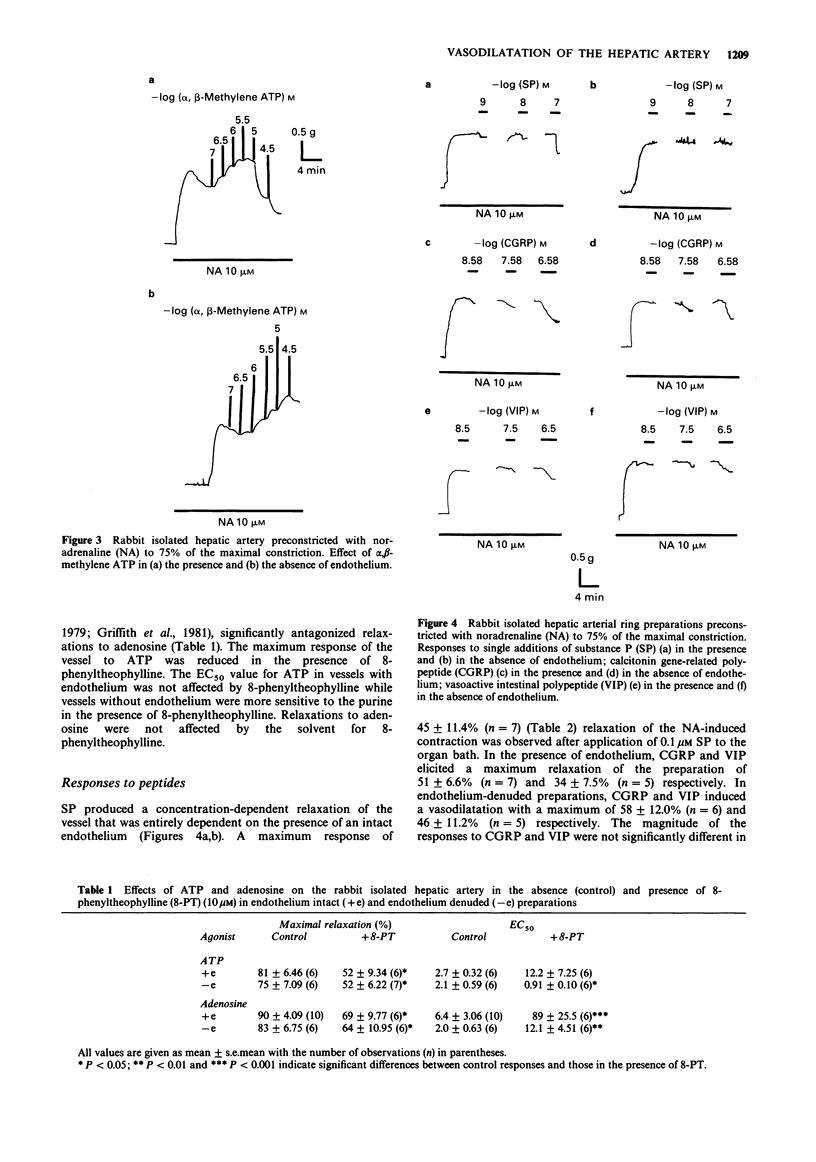
1. The isolated hepatic artery of the rabbit contracted to exogenously applied noradrenaline (NA). There was no significant difference in the maximal contraction or the EC50 value in vessels where the endothelium was present and in endothelium-denuded preparations. 2. Acetylcholine (ACh) induced a vasodilatation of vessels preconstricted with NA which was entirely dependent on the endothelium. 3. Adenosine 5'-triphosphate (ATP), 2-methylthio ATP, adenosine and sodium nitroprusside induced concentration-dependent, sustained relaxations of vessels in which tone had been induced with NA. The relaxation responses were not reduced after removal of the endothelium. 8-Phenyltheophylline antagonized the relaxation response produced by adenosine, but not that due to ATP at lower concentrations. The maximum response to ATP was reduced in the presence of 8-phenyltheophylline. 4. alpha,beta-Methylene ATP produced further contraction of vessels preconstricted with NA in both endothelium-denuded preparations and in vessels where the endothelium remained intact. 5. Immunohistochemical analysis was used to show the presence of nerve fibres containing substance P (SP), calcitonin gene-related peptide (CGRP) and vasoactive intestinal polypeptide (VIP) in the hepatic artery. Application of SP induced a concentration-dependent relaxation which was entirely dependent on the presence of an intact endothelium. CGRP and VIP, however, elicited concentration-dependent relaxations which were independent of the endothelium. 7. It is concluded that in the rabbit hepatic artery, responses to ACh are dependent on the presence of intact endothelium. P1-, P2x- and P2y-purinoceptors, mediating relaxation to adenosine, vasoconstriction to ATP and vasodilatation to ATP respectively, are located on vascular smooth muscle.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E. Measurements of oxygen consumption in smooth muscle. J Physiol. 1953 Oct;122(1):111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja F., Mathison R., Huggel H. Substance P-containing nerve fibres in large peripheral blood vessels of the rat. Cell Tissue Res. 1983;229(2):411–422. doi: 10.1007/BF00214982. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clapp L. H. Endothelial-dependent relaxant actions of carbachol and substance P in arterial smooth muscle. Br J Pharmacol. 1986 Apr;87(4):713–723. doi: 10.1111/j.1476-5381.1986.tb14589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J., Tippins J. R., Morris H. R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985 Jan 3;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Brizzolara A. L., Burnstock G. Evidence for noradrenergic-purinergic cotransmission in the hepatic artery of the rabbit. Br J Pharmacol. 1990 Apr;99(4):835–839. doi: 10.1111/j.1476-5381.1990.tb13016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. A pharmacological study of the rabbit saphenous artery in vitro: a vessel with a large purinergic contractile response to sympathetic nerve stimulation. Br J Pharmacol. 1987 Jan;90(1):111–120. doi: 10.1111/j.1476-5381.1987.tb16830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan B. A., Gerrity R. G., Schwartz C. J. Endothelial cell morphology in focal areas of in vivo Evans blue uptake in the young pig aorta. I. Quantitative light microscopic findings. Exp Mol Pathol. 1974 Aug;21(1):102–117. doi: 10.1016/0014-4800(74)90082-3. [DOI] [PubMed] [Google Scholar]
- Costa M., Buffa R., Furness J. B., Solcia E. Immunohistochemical localization of polypeptides in peripheral autonomic nerves using whole mount preparations. Histochemistry. 1980 Feb;65(2):157–165. doi: 10.1007/BF00493164. [DOI] [PubMed] [Google Scholar]
- D'Orléans-Juste P., Dion S., Mizrahi J., Regoli D. Effects of peptides and non-peptides on isolated arterial smooth muscles: role of endothelium. Eur J Pharmacol. 1985 Aug 7;114(1):9–21. doi: 10.1016/0014-2999(85)90515-1. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982 Oct;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles S. P., Said S. I. Vasoactive intestinal peptide as a neurotransmitter in the cerebral circulation. Eur J Pharmacol. 1982 Mar 12;78(3):371–374. doi: 10.1016/0014-2999(82)90041-3. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B. B., Hamel E., Jansen I., Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett. 1985 Jul 31;58(2):213–217. doi: 10.1016/0304-3940(85)90166-1. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Gulbenkian S., Wharton J., Jansen I., Polak J. M. Peptide-containing nerves in the rat femoral artery and vein. An immunocytochemical and vasomotor study. Blood Vessels. 1989;26(5):254–271. doi: 10.1159/000158775. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Substance P: immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. J Physiol. 1981 Sep;318:251–258. doi: 10.1113/jphysiol.1981.sp013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas A., Gazelius B., Edwall B., Theodorsson-Norheim E., Blomquist L., Lundberg J. M. VIP and noncholinergic vasodilatation in rabbit submandibular gland. Peptides. 1987 Jan-Feb;8(1):13–20. doi: 10.1016/0196-9781(87)90157-4. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Papka R. E., Della N. G., Costa M., Eskay R. L. Substance P-like immunoreactivity in nerves associated with the vascular system of guinea-pigs. Neuroscience. 1982 Feb;7(2):447–459. doi: 10.1016/0306-4522(82)90279-2. [DOI] [PubMed] [Google Scholar]
- Goehler L. E., Sternini C., Brecha N. C. Calcitonin gene-related peptide immunoreactivity in the biliary pathway and liver of the guinea-pig: distribution and colocalization with substance P. Cell Tissue Res. 1988 Jul;253(1):145–150. doi: 10.1007/BF00221749. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Martin W. Endothelium-dependent relaxation of the pig aorta: relationship to stimulation of 86Rb efflux from isolated endothelial cells. Br J Pharmacol. 1983 Jun;79(2):531–541. doi: 10.1111/j.1476-5381.1983.tb11028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S. G., Meghji P., Moody C. J., Burnstock G. 8-phenyltheophylline: a potent P1-purinoceptor antagonist. Eur J Pharmacol. 1981 Oct 15;75(1):61–64. doi: 10.1016/0014-2999(81)90346-0. [DOI] [PubMed] [Google Scholar]
- Hand J. M., Laravuso R. B., Will J. A. Relaxation of isolated guinea pig trachea, bronchi and pulmonary arteries produced by vasoactive intestinal peptide (VIP). Eur J Pharmacol. 1984 Feb 17;98(2):279–284. doi: 10.1016/0014-2999(84)90602-2. [DOI] [PubMed] [Google Scholar]
- Hanko J., Hardebo J. E., Kåhrström J., Owman C., Sundler F. Calcitonin gene-related peptide is present in mammalian cerebrovascular nerve fibres and dilates pial and peripheral arteries. Neurosci Lett. 1985 Jun 4;57(1):91–95. doi: 10.1016/0304-3940(85)90045-x. [DOI] [PubMed] [Google Scholar]
- Houston D. A., Burnstock G., Vanhoutte P. M. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987 May;241(2):501–506. [PubMed] [Google Scholar]
- Kawasaki H., Takasaki K., Saito A., Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988 Sep 8;335(6186):164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Burnstock G. ATP produces vasodilation via P1 purinoceptors and vasoconstriction via P2 purinoceptors in the isolated rabbit central ear artery. Blood Vessels. 1985;22(3):145–155. doi: 10.1159/000158592. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Burnstock G. Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur J Pharmacol. 1985 Apr 23;111(1):49–56. doi: 10.1016/0014-2999(85)90112-8. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Delbro D., Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985 Jan 2;107(2):161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Edvinsson L., Fahrenkrug J., Håkanson R., Owman C., Schaffalitzky de Muckadell O., Sundler F. Immunohistochemical localization of a vasodilatory polypeptide (VIP) in cerebrovascular nerves. Brain Res. 1976 Aug 27;113(2):400–404. doi: 10.1016/0006-8993(76)90951-3. [DOI] [PubMed] [Google Scholar]
- Lee T. J., Saito A., Berezin I. Vasoactive intestinal polypeptide-like substance: the potential transmitter for cerebral vasodilation. Science. 1984 May 25;224(4651):898–901. doi: 10.1126/science.6719122. [DOI] [PubMed] [Google Scholar]
- Lincoln J., Loesch A., Burnstock G. Localization of vasopressin, serotonin and angiotensin II in endothelial cells of the renal and mesenteric arteries of the rat. Cell Tissue Res. 1990 Feb;259(2):341–344. doi: 10.1007/BF00318457. [DOI] [PubMed] [Google Scholar]
- Liu S. F., McCormack D. G., Evans T. W., Barnes P. J. Evidence for two P2-purinoceptor subtypes in human small pulmonary arteries. Br J Pharmacol. 1989 Nov;98(3):1014–1020. doi: 10.1111/j.1476-5381.1989.tb14633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looareesuwan S., Phillips R. E., White N. J., Kietinun S., Karbwang J., Rackow C., Turner R. C., Warrell D. A. Quinine and severe falciparum malaria in late pregnancy. Lancet. 1985 Jul 6;2(8445):4–8. doi: 10.1016/s0140-6736(85)90056-x. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of hormones and neuropeptides by calmodulin. Biochemistry. 1983 Apr 12;22(8):1995–2001. doi: 10.1021/bi00277a040. [DOI] [PubMed] [Google Scholar]
- Martin W., Cusack N. J., Carleton J. S., Gordon J. L. Specificity of P2-purinoceptor that mediates endothelium-dependent relaxation of the pig aorta. Eur J Pharmacol. 1985 Feb 5;108(3):295–299. doi: 10.1016/0014-2999(85)90452-2. [DOI] [PubMed] [Google Scholar]
- Mathie R. T., Alexander B. The role of adenosine in the hyperaemic response of the hepatic artery to portal vein occlusion (the 'buffer response'). Br J Pharmacol. 1990 Jul;100(3):626–630. doi: 10.1111/j.1476-5381.1990.tb15857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson J. J., Burnstock G. Purine-mediated relaxation and constriction of isolated rabbit mesenteric artery are not endothelium-dependent. Eur J Pharmacol. 1985 Dec 3;118(3):221–229. doi: 10.1016/0014-2999(85)90132-3. [DOI] [PubMed] [Google Scholar]
- Mione M. C., Ralevic V., Burnstock G. Peptides and vasomotor mechanisms. Pharmacol Ther. 1990;46(3):429–468. doi: 10.1016/0163-7258(90)90027-y. [DOI] [PubMed] [Google Scholar]
- Murad F., Arnold W. P., Mittal C. K., Braughler J. M. Properties and regulation of guanylate cyclase and some proposed functions for cyclic GMP. Adv Cyclic Nucleotide Res. 1979;11:175–204. [PubMed] [Google Scholar]
- Peach M. J., Loeb A. L., Singer H. A., Saye J. Endothelium-derived vascular relaxing factor. Hypertension. 1985 May-Jun;7(3 Pt 2):I94–100. doi: 10.1161/01.hyp.7.3_pt_2.i94. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Mechanisms of adenosine triphosphate-, thrombin-, and trypsin-induced relaxation of rat thoracic aorta. Circ Res. 1984 Oct;55(4):468–479. doi: 10.1161/01.res.55.4.468. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Hayashi N., Kasahara A., Matsuda H., Fusamoto H., Sato N., Hillyard C. J., Girgis S., MacIntyre I., Emson P. C. Calcitonin gene-related peptide in the hepatic and splanchnic vascular systems of the rat. Hepatology. 1986 Jul-Aug;6(4):676–681. doi: 10.1002/hep.1840060423. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Stoclet J. C. Effect of vasoactive intestinal polypeptide (VIP) on cyclic AMP level and relaxation in rat isolated aorta. Eur J Pharmacol. 1985 Feb 26;109(2):275–279. doi: 10.1016/0014-2999(85)90430-3. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Stewart-Lee A., Burnstock G. Actions of tachykinins on the rabbit mesenteric artery: substance P and [Glp6,L-Pro9]SP6-11 are potent agonists for endothelial neurokinin-1 receptors. Br J Pharmacol. 1989 Aug;97(4):1218–1224. doi: 10.1111/j.1476-5381.1989.tb12581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddman R., Alumets J., Edvinsson L., Håkanson R., Sundler F. VIP nerve fibres around peripheral blood vessels. Acta Physiol Scand. 1981 May;112(1):65–70. doi: 10.1111/j.1748-1716.1981.tb06783.x. [DOI] [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Ekblad E., Håkanson R., Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. 1986 Aug;15(1):1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Rimele T. J. Role of the endothelium in the control of vascular smooth muscle function. J Physiol (Paris) 1982;78(7):681–686. [PubMed] [Google Scholar]


