Abstract
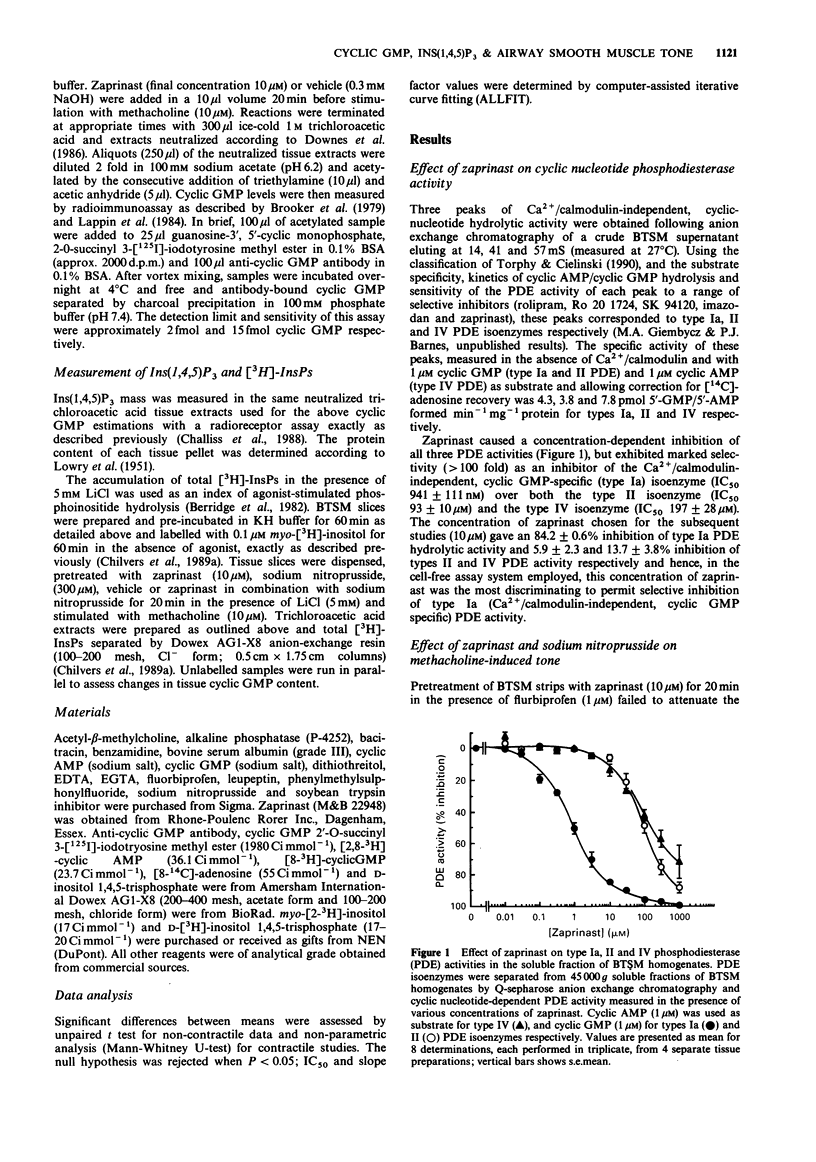
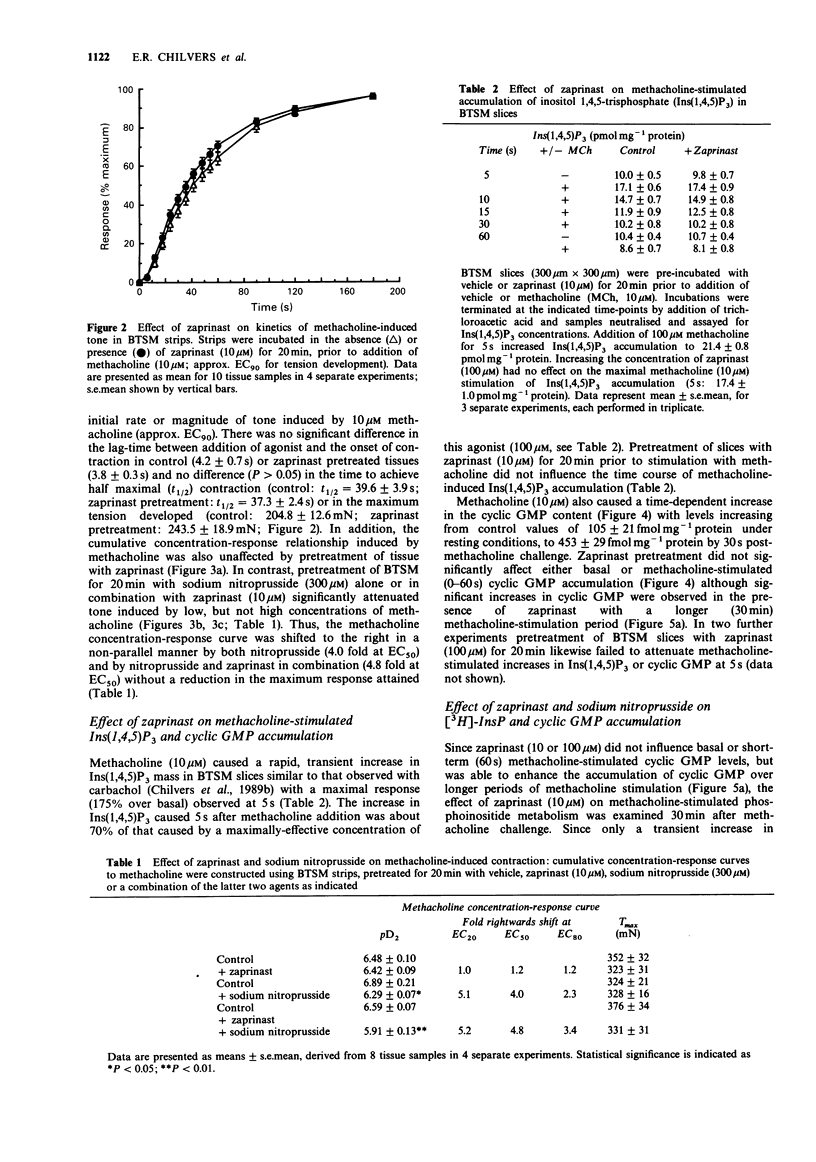
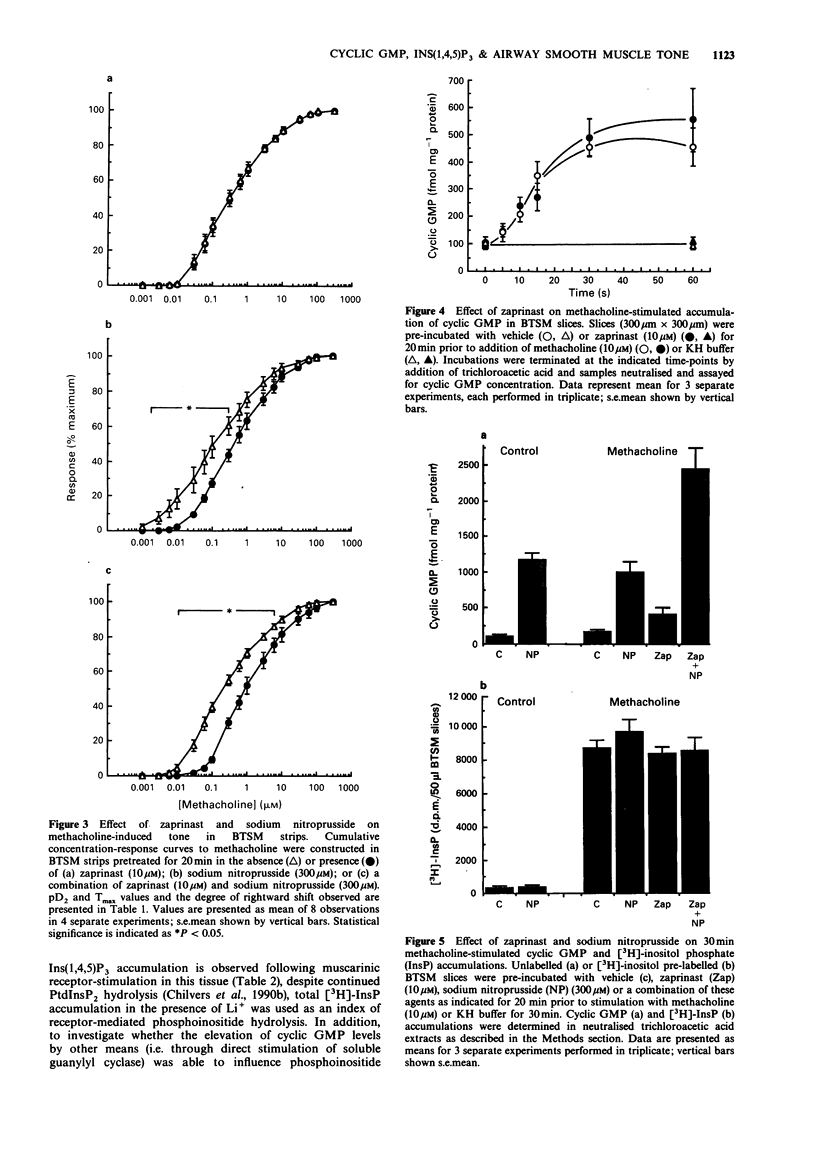
1. The effects of zaprinast (M&B 22948), a selective guanosine 3':5'-cyclic monophosphate (cyclic GMP) phosphodiesterase inhibitor, and sodium nitroprusside on cyclic GMP content, phosphoinositide hydrolysis and airway smooth muscle tone were examined in flurbiprofen pretreated bovine tracheal smooth muscle (BTSM). 2. Anion-exchange chromatography of the soluble fraction of BTSM homogenates resolved three peaks of Ca2+/calmodulin-independent phosphodiesterase (PDE) activity that corresponded to type Ia (cyclic GMP-specific, zaprinast-inhibitable), type II (cyclic GMP-stimulated) and type IV (Ro 20 1724-inhibitable) PDE isoenzymes. Zaprinast caused a selective inhibition of the type Ia PDE isoenzyme (IC50 0.94 microM) with respect to the type II and IV (IC50 s 93 microM and 197 microM respectively) isoenzymes. 3. Pretreatment of BTSM strips with zaprinast (10 microM) for 20 min affected neither the initial rate of force development, nor the resultant magnitude of contraction induced by methacholine (10 microM). In addition, zaprinast (10 microM; 20 min) did not affect the cumulative concentration-response relationship induced by methacholine. In contrast, sodium nitroprusside (300 microM) either alone, or in combination with zaprinast (10 microM), significantly attenuated tone induced by low, but not high concentrations of methacholine. This resulted in a non-parallel, rightwards shift of the methacholine concentration-response curves (nitroprusside: 4.0 fold; nitroprusside/zaprinast: 4.8 fold at the EC50 values), without a reduction in the maximum tone generated. 4. In BTSM slices, zaprinast (10 or 100 microM) did not influence basal or methacholine (10 microM)-stimulated cyclic GMP accumulation or inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) mass accumulation over a 60s incubation period, although it did significantly increase cyclic GMP content over longer (30 min) stimulation periods.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Ahlner J., Axelsson K. L., Karlsson J. G., Andersson R. G. Glyceryl trinitrate inhibits phosphatidylinositol hydrolysis and protein kinase C activity in bovine mesenteric artery. Life Sci. 1988;43(15):1241–1248. doi: 10.1016/0024-3205(88)90214-7. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Casnellie J. E., Greengard P. Guanosine 3':5'-cyclic monophosphate-dependent phosphorylation of endogenous substrate proteins in membranes of mammalian smooth muscle. Proc Natl Acad Sci U S A. 1974 May;71(5):1891–1895. doi: 10.1073/pnas.71.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Batty I. H., Nahorski S. R. Mass measurements of inositol(1,4,5)trisphosphate in rat cerebral cortex slices using a radioreceptor assay: effects of neurotransmitters and depolarization. Biochem Biophys Res Commun. 1988 Dec 15;157(2):684–691. doi: 10.1016/s0006-291x(88)80304-8. [DOI] [PubMed] [Google Scholar]
- Chilvers E. R., Barnes P. J., Nahorski S. R. Characterization of agonist-stimulated incorporation of myo-[3H]inositol into inositol phospholipids and [3H]inositol phosphate formation in tracheal smooth muscle. Biochem J. 1989 Sep 15;262(3):739–746. doi: 10.1042/bj2620739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilvers E. R., Batty I. H., Barnes P. J., Nahorski S. R. Formation of inositol polyphosphates in airway smooth muscle after muscarinic receptor stimulation. J Pharmacol Exp Ther. 1990 Feb;252(2):786–791. [PubMed] [Google Scholar]
- Chilvers E. R., Challiss R. A., Barnes P. J., Nahorski S. R. Mass changes of inositol(1,4,5)trisphosphate in trachealis muscle following agonist stimulation. Eur J Pharmacol. 1989 May 30;164(3):587–590. doi: 10.1016/0014-2999(89)90269-0. [DOI] [PubMed] [Google Scholar]
- Coburn R. F., Baron C. B. Coupling mechanisms in airway smooth muscle. Am J Physiol. 1990 Apr;258(4 Pt 1):L119–L133. doi: 10.1152/ajplung.1990.258.4.L119. [DOI] [PubMed] [Google Scholar]
- Degerman E., Belfrage P., Newman A. H., Rice K. C., Manganiello V. C. Purification of the putative hormone-sensitive cyclic AMP phosphodiesterase from rat adipose tissue using a derivative of cilostamide as a novel affinity ligand. J Biol Chem. 1987 Apr 25;262(12):5797–5807. [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felbel J., Trockur B., Ecker T., Landgraf W., Hofmann F. Regulation of cytosolic calcium by cAMP and cGMP in freshly isolated smooth muscle cells from bovine trachea. J Biol Chem. 1988 Nov 15;263(32):16764–16771. [PubMed] [Google Scholar]
- Fiscus R. R., Torphy T. J., Mayer S. E. Cyclic GMP-dependent protein kinase activation in canine tracheal smooth muscle by methacholine and sodium nitroprusside. Biochim Biophys Acta. 1984 Dec 11;805(4):382–392. doi: 10.1016/0167-4889(84)90022-3. [DOI] [PubMed] [Google Scholar]
- Gillespie P. G., Beavo J. A. Inhibition and stimulation of photoreceptor phosphodiesterases by dipyridamole and M&B 22,948. Mol Pharmacol. 1989 Nov;36(5):773–781. [PubMed] [Google Scholar]
- Hall I. P., Chilvers E. R. Inositol phosphates and airway smooth muscle. Pulm Pharmacol. 1989;2(3):113–120. doi: 10.1016/0952-0600(89)90034-3. [DOI] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Inhibition of histamine-stimulated inositol phospholipid hydrolysis by agents which increase cyclic AMP levels in bovine tracheal smooth muscle. Br J Pharmacol. 1989 Jun;97(2):603–613. doi: 10.1111/j.1476-5381.1989.tb11992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Modulation of carbachol-induced inositol phosphate formation in bovine tracheal smooth muscle by cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol. 1990 Apr 15;39(8):1357–1363. doi: 10.1016/0006-2952(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Hall I. P., Hill S. J. Beta-adrenoceptor stimulation inhibits histamine-stimulated inositol phospholipid hydrolysis in bovine tracheal smooth muscle. Br J Pharmacol. 1988 Dec;95(4):1204–1212. doi: 10.1111/j.1476-5381.1988.tb11757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Ito Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br J Pharmacol. 1985 Sep;86(1):191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Kohse K. P., Chang C. H., Ikebe T., Murad F. Mechanism of cyclic GMP inhibition of inositol phosphate formation in rat aorta segments and cultured bovine aortic smooth muscle cells. J Biol Chem. 1990 Jan 25;265(3):1268–1273. [PubMed] [Google Scholar]
- Hogaboom G. K., Emler C. A., Butcher F. R., Fedan J. S. Concerted phosphorylation of endogenous tracheal smooth muscle membrane proteins by Ca2+ . calmodulin-, cyclic GMP- and cyclic AMP-dependent protein kinases. FEBS Lett. 1982 Mar 22;139(2):309–312. doi: 10.1016/0014-5793(82)80877-6. [DOI] [PubMed] [Google Scholar]
- Ishii K., Murad F. ANP relaxes bovine tracheal smooth muscle and increases cGMP. Am J Physiol. 1989 Mar;256(3 Pt 1):C495–C500. doi: 10.1152/ajpcell.1989.256.3.C495. [DOI] [PubMed] [Google Scholar]
- Kajikuri J., Kuriyama H. Inhibitory action of alpha-human atrial natriuretic peptide on noradrenaline-induced synthesis of myo-inositol 1,4,5-trisphosphate in the smooth muscle cells of rabbit aorta. Br J Pharmacol. 1990 Mar;99(3):536–540. doi: 10.1111/j.1476-5381.1990.tb12964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S., Murad F. Regulation of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol. 1977 Mar;13(2):330–341. [PubMed] [Google Scholar]
- Kim U. H., Kim J. W., Rhee S. G. Phosphorylation of phospholipase C-gamma by cAMP-dependent protein kinase. J Biol Chem. 1989 Dec 5;264(34):20167–20170. [PubMed] [Google Scholar]
- Kobayashi S., Kanaide H., Nakamura M. Cytosolic-free calcium transients in cultured vascular smooth muscle cells: microfluorometric measurements. Science. 1985 Aug 9;229(4713):553–556. doi: 10.1126/science.3927484. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langlands J. M., Rodger I. W., Diamond J. The effect of M&B 22948 on methacholine- and histamine-induced contraction and inositol 1,4,5-trisphosphate levels in guinea-pig tracheal tissue. Br J Pharmacol. 1989 Oct;98(2):336–338. doi: 10.1111/j.1476-5381.1989.tb12600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin D., Riches D. W., Damerau B., Whaley K. Cyclic nucleotides and their relationship to complement-component-C2 synthesis by human monocytes. Biochem J. 1984 Sep 1;222(2):477–486. doi: 10.1042/bj2220477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison J. M., Brown J. K. Differential inhibitory effects of forskolin, isoproterenol, and dibutyryl cyclic adenosine monophosphate on phosphoinositide hydrolysis in canine tracheal smooth muscle. J Clin Invest. 1988 Oct;82(4):1462–1465. doi: 10.1172/JCI113752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., de Lanerolle P., Lincoln T. M., Adelstein R. S. Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase. J Biol Chem. 1984 Jul 10;259(13):8429–8436. [PubMed] [Google Scholar]
- Parks T. P., Nairn A. C., Greengard P., Jamieson J. D. The cyclic nucleotide-dependent phosphorylation of aortic smooth muscle membrane proteins. Arch Biochem Biophys. 1987 Jun;255(2):361–371. doi: 10.1016/0003-9861(87)90404-8. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem J. 1988 May 15;252(1):269–273. doi: 10.1042/bj2520269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M. Cyclic guanosine monophosphate inhibition of contraction may be mediated through inhibition of phosphatidylinositol hydrolysis in rat aorta. Circ Res. 1986 Mar;58(3):407–410. doi: 10.1161/01.res.58.3.407. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Passonneau J. V. Cyclic AMP-mediated induction of the cyclic AMP phosphodiesterase of C-6 glioma cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3844–3848. doi: 10.1073/pnas.71.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. J., Hamel L. T., Perrone M. H., Bentley R. G., Bushover C. R., Evans D. B. Differential pharmacologic sensitivity of cyclic nucleotide phosphodiesterase isozymes isolated from cardiac muscle, arterial and airway smooth muscle. Eur J Pharmacol. 1988 May 20;150(1-2):85–94. doi: 10.1016/0014-2999(88)90753-4. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Torphy T. J., Cieslinski L. B. Characterization and selective inhibition of cyclic nucleotide phosphodiesterase isozymes in canine tracheal smooth muscle. Mol Pharmacol. 1990 Feb;37(2):206–214. [PubMed] [Google Scholar]
- Torphy T. J., Freese W. B., Rinard G. A., Brunton L. L., Mayer S. E. Cyclic nucleotide-dependent protein kinases in airway smooth muscle. J Biol Chem. 1982 Oct 10;257(19):11609–11616. [PubMed] [Google Scholar]
- Torphy T. J., Zheng C., Peterson S. M., Fiscus R. R., Rinard G. A., Mayer S. E. Inhibitory effect of methacholine on drug-induced relaxation, cyclic AMP accumulation, and cyclic AMP-dependent protein kinase activation in canine tracheal smooth muscle. J Pharmacol Exp Ther. 1985 May;233(2):409–417. [PubMed] [Google Scholar]
- VAN ROSSUM J. M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- Zwiller J., Revel M. O., Malviya A. N. Protein kinase C catalyzes phosphorylation of guanylate cyclase in vitro. J Biol Chem. 1985 Feb 10;260(3):1350–1353. [PubMed] [Google Scholar]


