Abstract
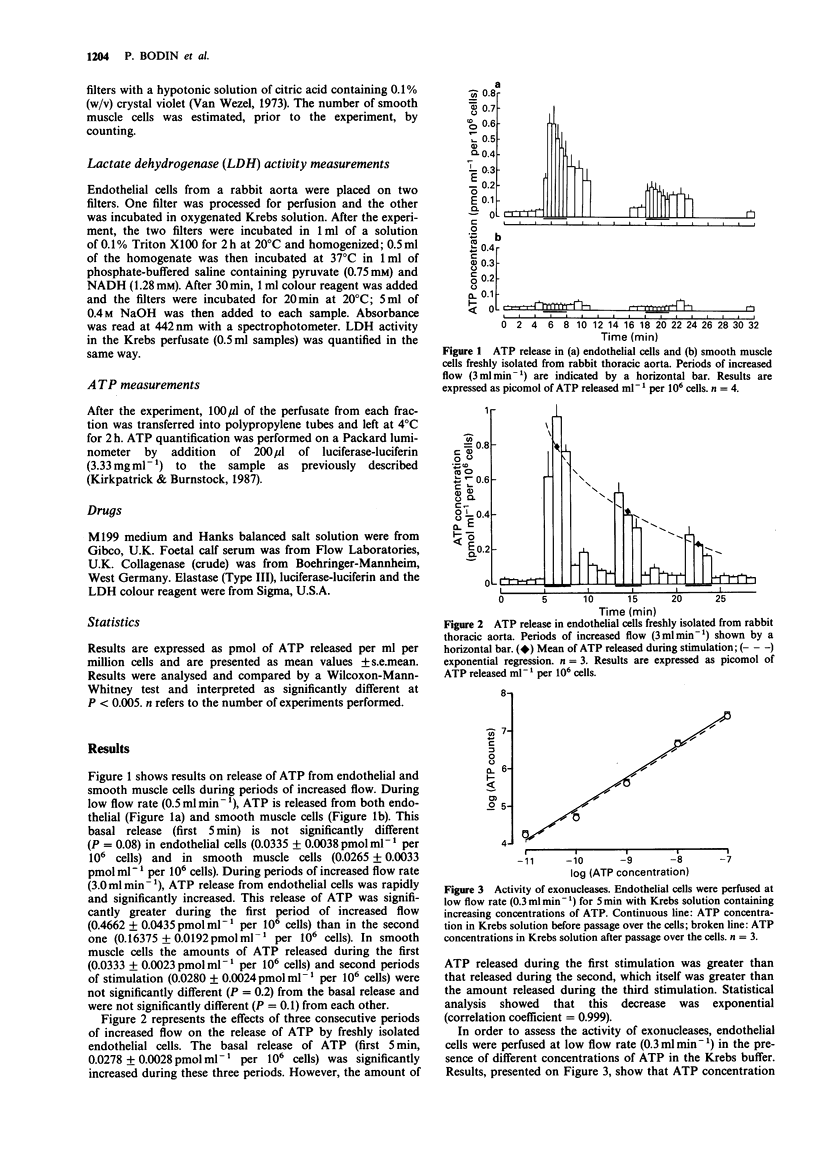
1. Freshly harvested smooth muscle cells and endothelial cells isolated from the rabbit aorta were perfused (0.5 ml min-1) and stimulated twice by an increase of flow rate (3.0 ml min-1) in order to compare their ability to release adenosine 5'-triphosphate (ATP). 2. In smooth muscle cells, the basal release of ATP (0.0265 +/- 0.0033 pmol ml-1 per 10(6) cells) was not increased during periods of increased flow (P = 0.2). 3. In endothelial cells, the concentration of ATP in the perfusate during periods of low flow (0.0335 +/- 0.0038 pmol ml-1 per 10(6) cells) was significantly increased by 14 times and 5 times during the first and second periods of increased flow, respectively. 4. The release of ATP by endothelial cells did not appear to be caused by the lysis of cells during the period of increased flow because it can be reproduced several times and because there was no difference between lactate dehydrogenase activity in perfused cells and that in non-perfused cells. 5. These results show that, of the two major cell types of the vascular wall, only endothelial cells react to shear stress by releasing ATP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Houston D. A., Burnstock G., Vanhoutte P. M. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987 May;241(2):501–506. [PubMed] [Google Scholar]
- Hull S. S., Jr, Kaiser L., Jaffe M. D., Sparks H. V., Jr Endothelium-dependent flow-induced dilation of canine femoral and saphenous arteries. Blood Vessels. 1986;23(4-5):183–198. doi: 10.1159/000158641. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K., Burnstock G. Sympathetic nerve-mediated release of ATP from the guinea-pig vas deferens is unaffected by reserpine. Eur J Pharmacol. 1987 Jun 19;138(2):207–214. doi: 10.1016/0014-2999(87)90434-1. [DOI] [PubMed] [Google Scholar]
- Levitt B., Westfall D. P. Factors influencing the release of purines and norepinephrine in the rabbit portal vein. Blood Vessels. 1982;19(1):30–40. doi: 10.1159/000158371. [DOI] [PubMed] [Google Scholar]
- Milner P., Bodin P., Loesch A., Burnstock G. Rapid release of endothelin and ATP from isolated aortic endothelial cells exposed to increased flow. Biochem Biophys Res Commun. 1990 Jul 31;170(2):649–656. doi: 10.1016/0006-291x(90)92141-l. [DOI] [PubMed] [Google Scholar]
- Milner P., Kirkpatrick K. A., Ralevic V., Toothill V., Pearson J., Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc Biol Sci. 1990 Sep 22;241(1302):245–248. doi: 10.1098/rspb.1990.0092. [DOI] [PubMed] [Google Scholar]
- Paddle B. M., Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974;11(3):110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Hutchings A., Gordon J. L. Uptake and metabolism of adenosine by pig aortic endothelial and smooth-muscle cells in culture. Biochem J. 1978 Feb 15;170(2):265–271. doi: 10.1042/bj1700265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Sedaa K. O., Bjur R. A., Shinozuka K., Westfall D. P. Nerve and drug-induced release of adenine nucleosides and nucleotides from rabbit aorta. J Pharmacol Exp Ther. 1990 Mar;252(3):1060–1067. [PubMed] [Google Scholar]
- Su C. Neurogenic release of purine compounds in blood vessels. J Pharmacol Exp Ther. 1975 Oct;195(1):159–166. [PubMed] [Google Scholar]
- Westfall D. P., Sedaa K., Bjur R. A. Release of endogenous ATP from rat caudal artery. Blood Vessels. 1987;24(3):125–127. doi: 10.1159/000158684. [DOI] [PubMed] [Google Scholar]
- White T. D., Chaudhry A., Vohra M. M., Webb D., Leslie R. A. Characteristics of P2 (nucleotide) receptors mediating contraction and relaxation of rat aortic strips: possible physiological relevance. Eur J Pharmacol. 1985 Nov 26;118(1-2):37–44. doi: 10.1016/0014-2999(85)90660-0. [DOI] [PubMed] [Google Scholar]
- White T. D. Role of adenine compounds in autonomic neurotransmission. Pharmacol Ther. 1988;38(2):129–168. doi: 10.1016/0163-7258(88)90095-2. [DOI] [PubMed] [Google Scholar]


