Abstract
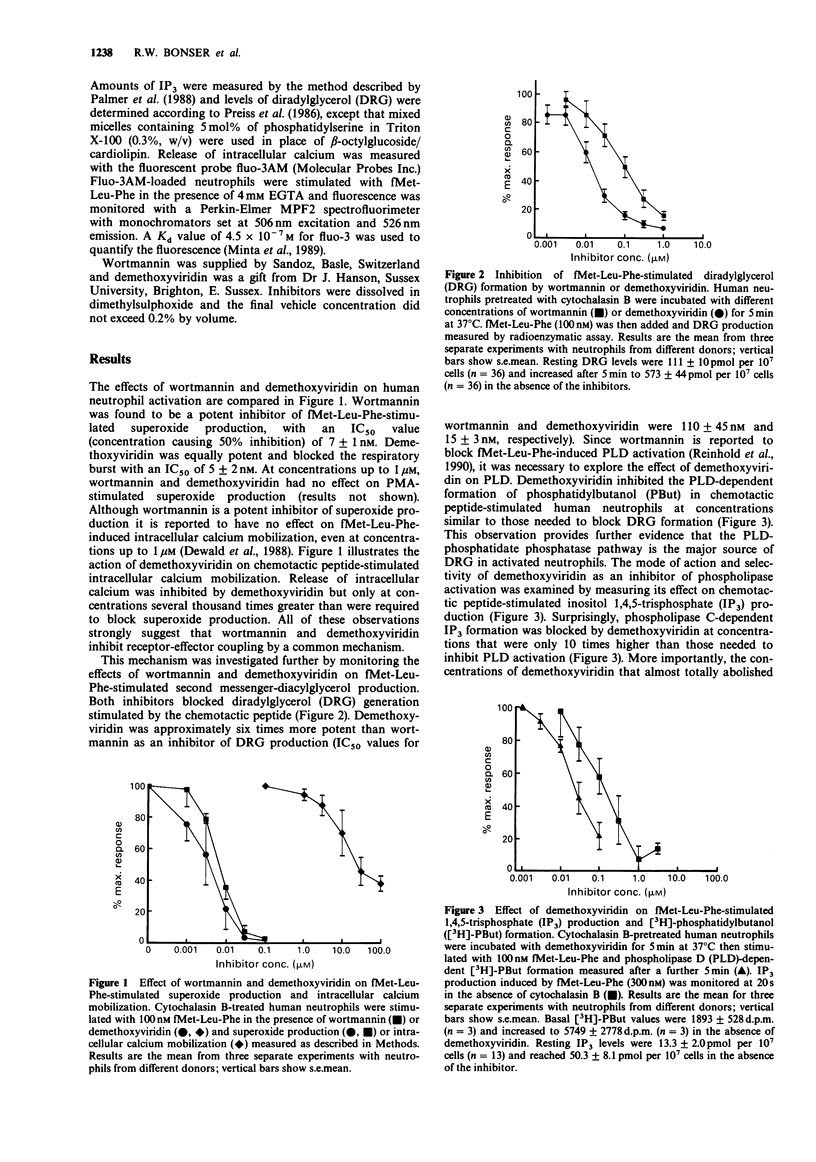
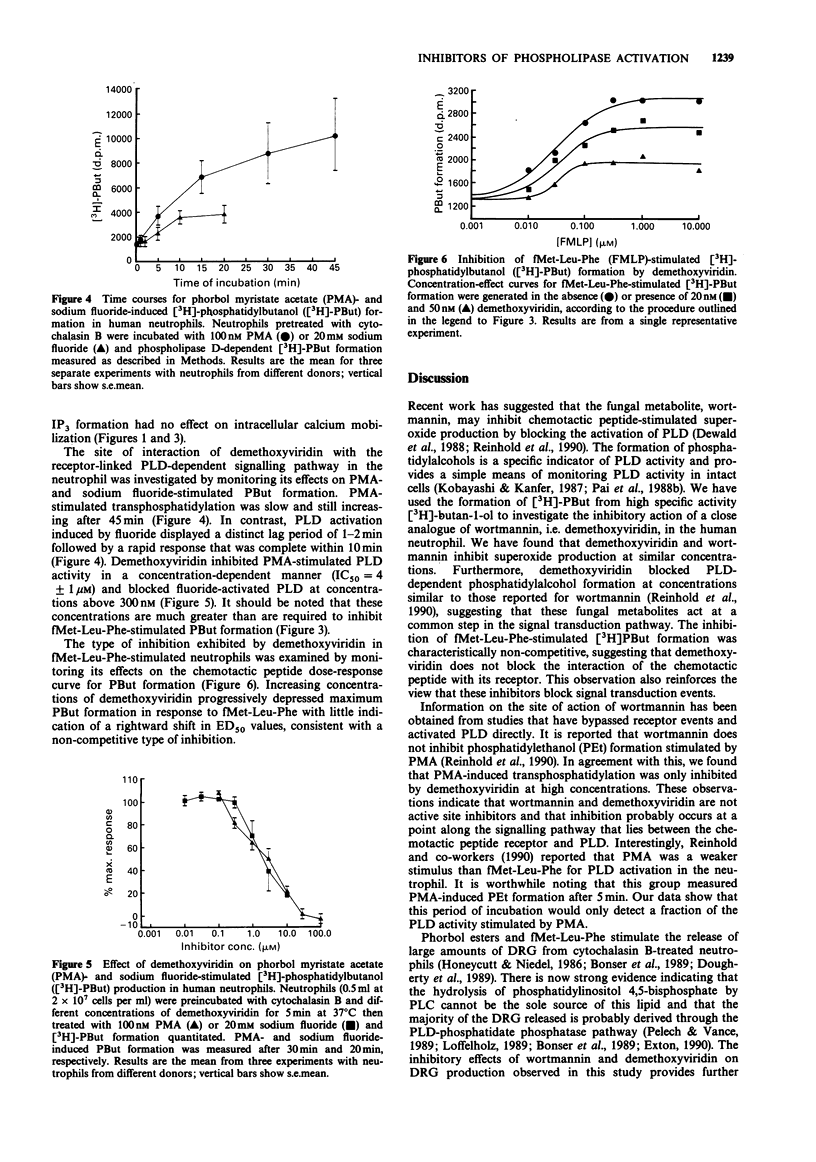
1. The fungal metabolite, wortmannin, has recently been shown to inhibit fMet-Leu-Phe-stimulated superoxide production and phospholipase D (PLD) activation in the human neutrophil. 2. We have found that a close structural analogue of wortmannin, demethoxyviridin, has a similar inhibitory profile but in addition blocks phosphatidylinositol 4,5-bisphosphate-specific phospholipase C and hence inositol 1,4,5-trisphosphate (IP3) formation. 3. Inhibition of fMet-Leu-Phe-stimulated PLD by demethoxyviridin was characteristically non-competitive (IC50 = 31 +/- 10 nM). 4. Inhibition of fMet-Leu-Phe-stimulation IP3 formation required concentrations almost 10 times higher (IC50 = 250 +/- 130 nM). 5. Surprisingly, demethoxyviridin only inhibited fMet-Leu-Phe-induced intracellular calcium mobilization at concentrations 100 times greater than those needed to block IP3 formation. 6. Demethoxyviridin also inhibited PLD activation induced by sodium fluoride or phorbol myristate acetate (PMA) but the concentrations required were 100 times those needed to block fMet-Leu-Phe-stimulated PLD. 7. These observations support the contention that PLD plays an important role in signal transduction in the human neutrophil and indicate that wortmannin and demethoxyviridin inhibit PLD activation at a common step in the signalling pathway. 8. Furthermore, these results suggest that demethoxyviridin may block the interaction between the chemotactic peptide receptor and a GTP-binding protein that is intimately involved in PLD activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthes J. C., Eckel S., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D in homogenates from HL-60 granulocytes: implications of calcium and G protein control. Biochem Biophys Res Commun. 1989 Aug 30;163(1):657–664. doi: 10.1016/0006-291x(89)92187-6. [DOI] [PubMed] [Google Scholar]
- Ben-Av P., Liscovitch M. Phospholipase D activation by the mitogens platelet-derived growth factor and 12-O-tetradecanoylphorbol 13-acetate in NIH-3T3 cells. FEBS Lett. 1989 Dec 18;259(1):64–66. doi: 10.1016/0014-5793(89)81495-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Pai J. K., Mullmann T. J., Egan R. W., Siegel M. I. Regulation of phospholipase D in HL-60 granulocytes. Activation by phorbol esters, diglyceride, and calcium ionophore via protein kinase- independent mechanisms. J Biol Chem. 1989 May 25;264(15):9069–9076. [PubMed] [Google Scholar]
- Bocckino S. B., Wilson P. B., Exton J. H. Ca2+-mobilizing hormones elicit phosphatidylethanol accumulation via phospholipase D activation. FEBS Lett. 1987 Dec 10;225(1-2):201–204. doi: 10.1016/0014-5793(87)81157-2. [DOI] [PubMed] [Google Scholar]
- Bonser R. W., Thompson N. T., Randall R. W., Garland L. G. Phospholipase D activation is functionally linked to superoxide generation in the human neutrophil. Biochem J. 1989 Dec 1;264(2):617–620. doi: 10.1042/bj2640617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Thelen M., Baggiolini M. Two transduction sequences are necessary for neutrophil activation by receptor agonists. J Biol Chem. 1988 Nov 5;263(31):16179–16184. [PubMed] [Google Scholar]
- Dougherty R. W., Dubay G. R., Niedel J. E. Dynamics of the diradylglycerol responses of stimulated phagocytes. J Biol Chem. 1989 Jul 5;264(19):11263–11269. [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Gabig T. G., English D., Akard L. P., Schell M. J. Regulation of neutrophil NADPH oxidase activation in a cell-free system by guanine nucleotides and fluoride. Evidence for participation of a pertussis and cholera toxin-insensitive G protein. J Biol Chem. 1987 Feb 5;262(4):1685–1690. [PubMed] [Google Scholar]
- Gelas P., Ribbes G., Record M., Terce F., Chap H. Differential activation by fMet-Leu-Phe and phorbol ester of a plasma membrane phosphatidylcholine-specific phospholipase D in human neutrophil. FEBS Lett. 1989 Jul 17;251(1-2):213–218. doi: 10.1016/0014-5793(89)81457-7. [DOI] [PubMed] [Google Scholar]
- Gruchalla R. S., Dinh T. T., Kennerly D. A. An indirect pathway of receptor-mediated 1,2-diacylglycerol formation in mast cells. I. IgE receptor-mediated activation of phospholipase D. J Immunol. 1990 Mar 15;144(6):2334–2342. [PubMed] [Google Scholar]
- Harris R. A., Schmidt J., Hitzemann B. A., Hitzemann R. J. Phosphatidate as a molecular link between depolarization and neurotransmitter release in the brain. Science. 1981 Jun 12;212(4500):1290–1291. doi: 10.1126/science.7233220. [DOI] [PubMed] [Google Scholar]
- Honeycutt P. J., Niedel J. E. Cytochalasin B enhancement of the diacylglycerol response in formyl peptide-stimulated neutrophils. J Biol Chem. 1986 Dec 5;261(34):15900–15905. [PubMed] [Google Scholar]
- Imagawa W., Bandyopadhyay G. K., Wallace D., Nandi S. Phospholipids containing polyunsaturated fatty acyl groups are mitogenic for normal mouse mammary epithelial cells in serum-free primary cell culture. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4122–4126. doi: 10.1073/pnas.86.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Stimulation of phosphatidylinositol 4,5-bisphosphate phospholipase C activity by phosphatidic acid. Arch Biochem Biophys. 1989 Feb 1;268(2):516–524. doi: 10.1016/0003-9861(89)90318-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Kanfer J. N. Phosphatidylethanol formation via transphosphatidylation by rat brain synaptosomal phospholipase D. J Neurochem. 1987 May;48(5):1597–1603. doi: 10.1111/j.1471-4159.1987.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Liscovitch M. Phosphatidylethanol biosynthesis in ethanol-exposed NG108-15 neuroblastoma X glioma hybrid cells. Evidence for activation of a phospholipase D phosphatidyl transferase activity by protein kinase C. J Biol Chem. 1989 Jan 25;264(3):1450–1456. [PubMed] [Google Scholar]
- Löffelholz K. Receptor regulation of choline phospholipid hydrolysis. A novel source of diacylglycerol and phosphatidic acid. Biochem Pharmacol. 1989 May 15;38(10):1543–1549. doi: 10.1016/0006-2952(89)90299-2. [DOI] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Phosphatidic acid mimics the muscarinic action of acetylcholine in cultured bovine chromaffin cells. FEBS Lett. 1983 Feb 7;152(1):62–66. doi: 10.1016/0014-5793(83)80482-7. [DOI] [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Stimulation of phosphatidic acid of calcium influx and cyclic GMP synthesis in neuroblastoma cells. J Biol Chem. 1981 Nov 10;256(21):10945–10948. [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Activation of phospholipase D by chemotactic peptide in HL-60 granulocytes. Biochem Biophys Res Commun. 1988 Jan 15;150(1):355–364. doi: 10.1016/0006-291x(88)90528-1. [DOI] [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D catalyzes phospholipid metabolism in chemotactic peptide-stimulated HL-60 granulocytes. J Biol Chem. 1988 Sep 5;263(25):12472–12477. [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Qian Z., Drewes L. R. Muscarinic acetylcholine receptor regulates phosphatidylcholine phospholipase D in canine brain. J Biol Chem. 1989 Dec 25;264(36):21720–21724. [PubMed] [Google Scholar]
- Randall R. W., Bonser R. W., Thompson N. T., Garland L. G. A novel and sensitive assay for phospholipase D in intact cells. FEBS Lett. 1990 May 7;264(1):87–90. doi: 10.1016/0014-5793(90)80772-b. [DOI] [PubMed] [Google Scholar]
- Reinhold S. L., Prescott S. M., Zimmerman G. A., McIntyre T. M. Activation of human neutrophil phospholipase D by three separable mechanisms. FASEB J. 1990 Feb 1;4(2):208–214. doi: 10.1096/fasebj.4.2.2105252. [DOI] [PubMed] [Google Scholar]
- Rubin R. Phosphatidylethanol formation in human platelets: evidence for thrombin-induced activation of phospholipase D. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1090–1096. doi: 10.1016/s0006-291x(88)80744-7. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateson J. E., Randall R. W., Reynolds C. H., Jackson W. P., Bhattacherjee P., Salmon J. A., Garland L. G. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: biochemical assessment in vitro and ex vivo. Br J Pharmacol. 1988 Jun;94(2):528–539. doi: 10.1111/j.1476-5381.1988.tb11557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettenborn C. S., Mueller G. C. 12-O-tetradecanoylphorbol-13-acetate activates phosphatidylethanol and phosphatidylglycerol synthesis by phospholipase D in cell lysates. Biochem Biophys Res Commun. 1988 Aug 30;155(1):249–255. doi: 10.1016/s0006-291x(88)81076-3. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]


