Abstract
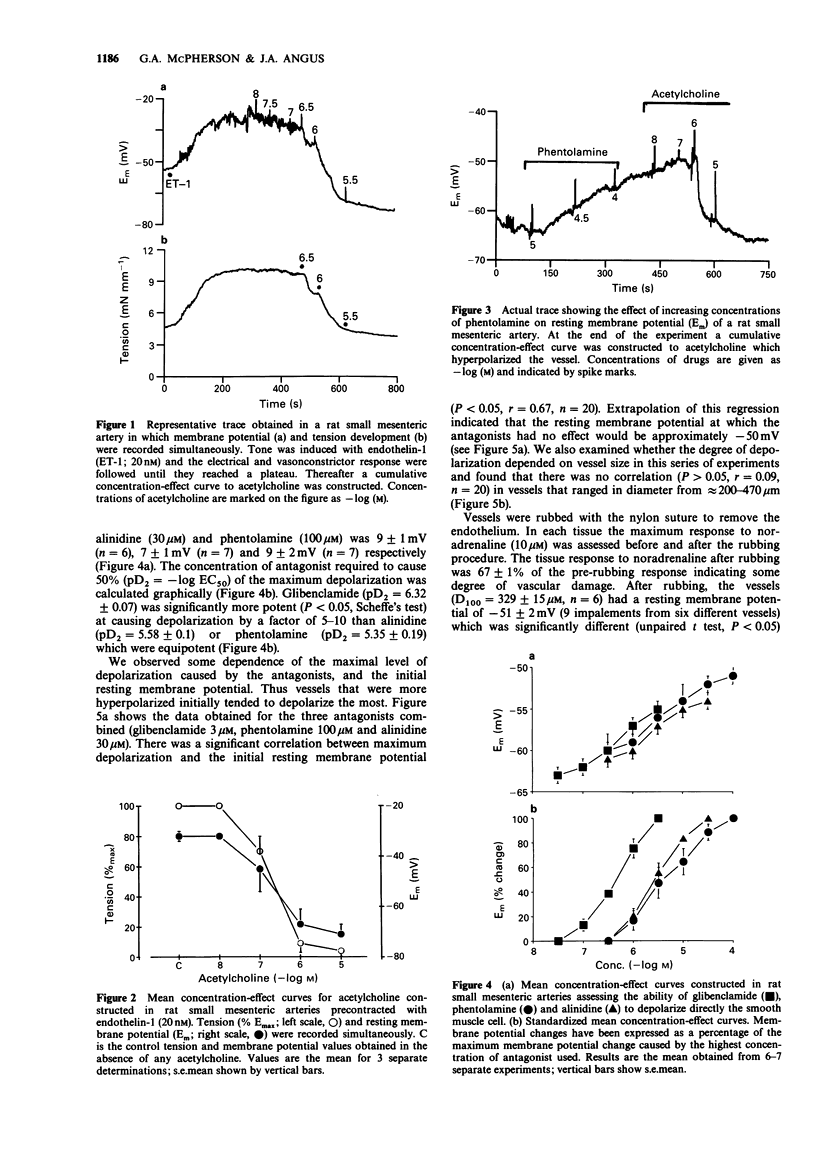
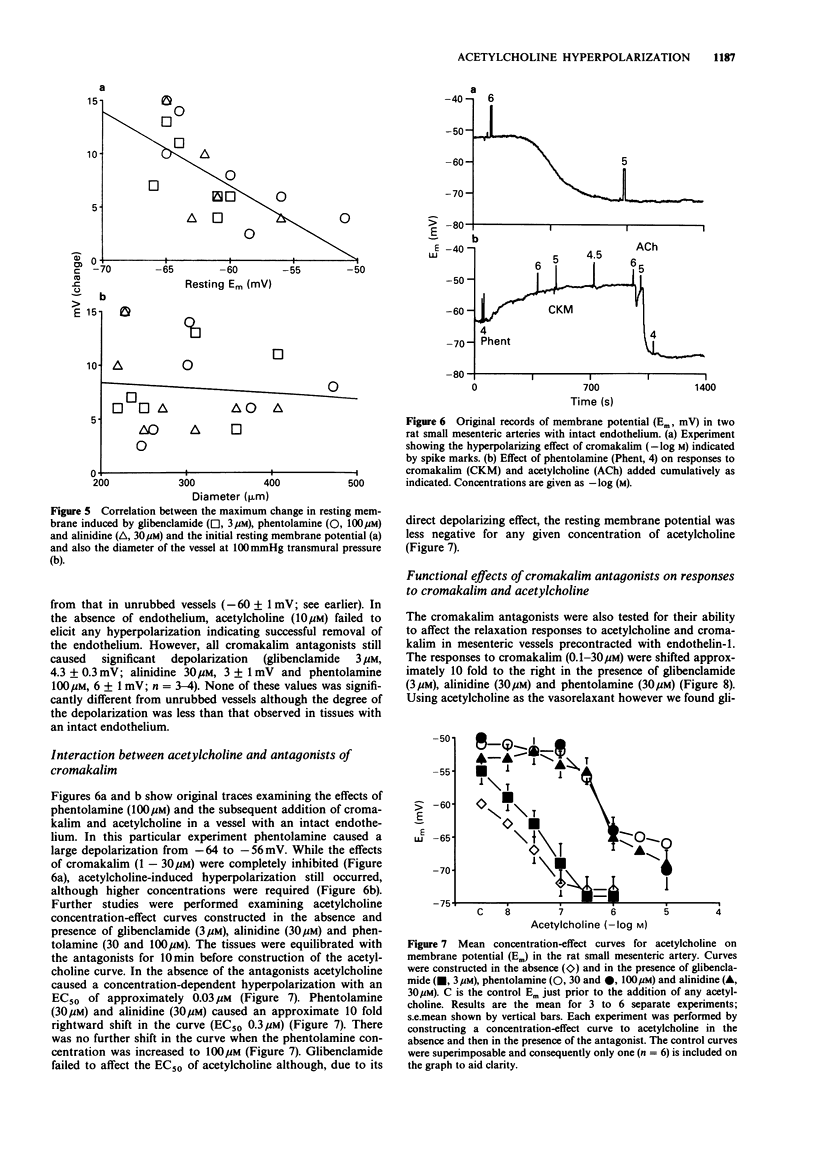
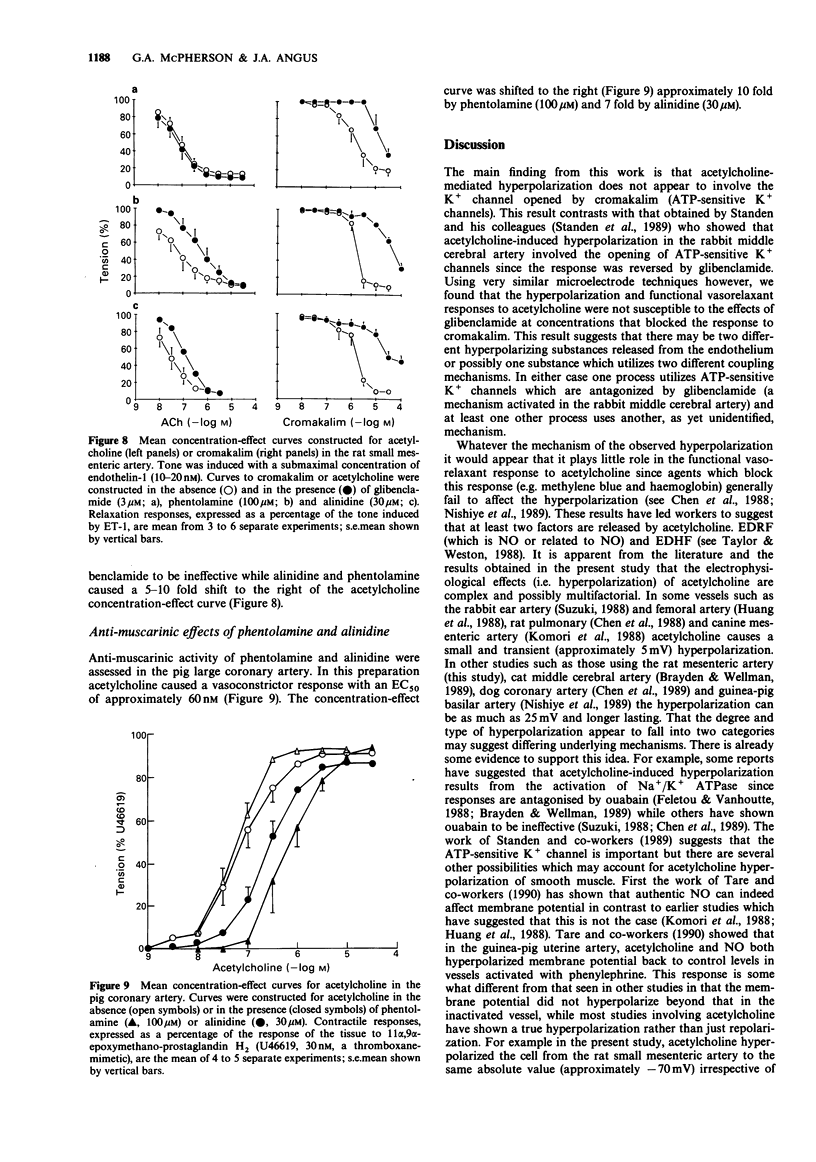
1. Acetylcholine causes a concentration-dependent hyperpolarization of the rat small mesenteric artery (diameter at 100 mmHg, 200-400 microns). In the absence of tone the average potential change was from approximately -60 to -75 mV. In the presence of tone induced by endothelin-1 (20 nM), acetylcholine caused vasorelaxation in association with a marked hyperpolarization; from approximately -32 to -71 mV. 2. A number of compounds known to antagonize the actions of cromakalim were tested for their ability to block responses to acetylcholine. Glibenclamide (0.1-3 microM), phentolamine (10-100 microM) and alinidine (1-30 microM) caused a concentration-dependent depolarization of the rat small mesenteric artery which was not dependent on an intact endothelium. Glibenclamide was approximately 10 times more potent than either phentolamine or alinidine, a similar ratio to their potency as antagonists of cromakalim. 3. In the presence of concentrations of the cromakalim antagonists which functionally inhibited responses to cromakalim, only phentolamine and alinidine had a significant effect on the hyperpolarization and functional responses to acetylcholine. Glibenclamide was without effect at the concentrations used. 4. Experiments on pig coronary artery, where acetylcholine causes vasoconstrictor responses, showed that phentolamine and alinidine have some anti-muscarinic activity which could account for their ability to affect vasorelaxant/hyperpolarization responses to acetylcholine in the rat small mesenteric artery. 5. The results suggest that the acetylcholine-mediated hyperpolarization observed in the rat small mesenteric artery does not involve K+ channels opened by cromakalim. This finding differs from other studies performed on the rabbit middle cerebral artery which show hyperpolarizing responses to acetycholine to be glibenclamide-sensitive.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Broughton A., Mulvany M. J. Role of alpha-adrenoceptors in constrictor responses of rat, guinea-pig and rabbit small arteries to neural activation. J Physiol. 1988 Sep;403:495–510. doi: 10.1113/jphysiol.1988.sp017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Cocks T. M. Endothelium-derived relaxing factor. Pharmacol Ther. 1989;41(1-2):303–352. doi: 10.1016/0163-7258(89)90112-5. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Wellman G. C. Endothelium-dependent dilation of feline cerebral arteries: role of membrane potential and cyclic nucleotides. J Cereb Blood Flow Metab. 1989 Jun;9(3):256–263. doi: 10.1038/jcbfm.1989.42. [DOI] [PubMed] [Google Scholar]
- Brunner F., Kukovetz W. R. Binding of two specific bradycardic agents, alinidine and AQ-A 39, to muscarinic receptors of guinea pig atria and ventricle. J Cardiovasc Pharmacol. 1988 Feb;11(2):222–229. [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Chen G., Hashitani H., Suzuki H. Endothelium-dependent relaxation and hyperpolarization of canine coronary artery smooth muscles in relation to the electrogenic Na-K pump. Br J Pharmacol. 1989 Nov;98(3):950–956. doi: 10.1111/j.1476-5381.1989.tb14625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Busse R., Bassenge E. Endothelium-dependent hyperpolarization of smooth muscle cells in rabbit femoral arteries is not mediated by EDRF (nitric oxide). Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):438–442. doi: 10.1007/BF00172124. [DOI] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- McPherson G. A., Angus J. A. Characterization of responses to cromakalim and pinacidil in smooth and cardiac muscle by use of selective antagonists. Br J Pharmacol. 1990 Jun;100(2):201–206. doi: 10.1111/j.1476-5381.1990.tb15782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson G. A., Angus J. A. Phentolamine and structurally related compounds selectively antagonize the vascular actions of the K+ channel opener, cromromakalim. Br J Pharmacol. 1989 Jul;97(3):941–949. doi: 10.1111/j.1476-5381.1989.tb12035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nishiye E., Nakao K., Itoh T., Kuriyama H. Factors inducing endothelium-dependent relaxation in the guinea-pig basilar artery as estimated from the actions of haemoglobin. Br J Pharmacol. 1989 Mar;96(3):645–655. doi: 10.1111/j.1476-5381.1989.tb11864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Suzuki H. The electrogenic Na-K pump does not contribute to endothelium-dependent hyperpolarization in the rabbit ear artery. Eur J Pharmacol. 1988 Nov 1;156(2):295–297. doi: 10.1016/0014-2999(88)90337-8. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]



