Abstract
1. The actions of oximes and related compounds on the nicotinic acetylcholine receptor ion channel at the adult mouse muscle endplate were investigated by use of single-channel recording techniques. The aim of the study was to determine whether the channel-blocking properties of the compounds could contribute to their therapeutic effectiveness against soman poisoning in vitro. 2. Therapeutic effectiveness was assessed in guinea-pig phrenic nerve-hemidiaphragm preparations by measuring the degree of recovery of neuromuscular function produced by the compounds following poisoning by soman. A number of the compounds, including some which lacked the oxime group, produced a significant recovery of neuromuscular function which was unrelated to acetylcholinesterase (AChE) reactivation; this was reversed by washing off the compound, and was therefore attributed to a direct pharmacological action on the muscle. 3. Single channel recordings showed that some of the compounds blocked open nicotinic receptor ion channels in preparations of mouse muscle fibres. The compounds which showed the greatest direct pharmacological actions in diaphragms produce a very fast, flickering blockade of the channels. Several quantitative measures of channel-blocking activity correlated very well with the direct pharmacological action. Furthermore, for two compounds studied in greater detail, the direct action and channel-blocking showed similar concentration-response relationships. 4. The results of this study indicate that the direct pharmacological action of oximes and their analogues against neuromuscular blockade by soman in vitro is due to their channel-blocking activity. The direct action does not correlate well with protection against soman poisoning in vivo, however, which suggests that additional non-reactivating properties of these compounds, at sites other than the neuromuscular junction, may also be important for their therapeutic effectiveness.
Full text
PDF


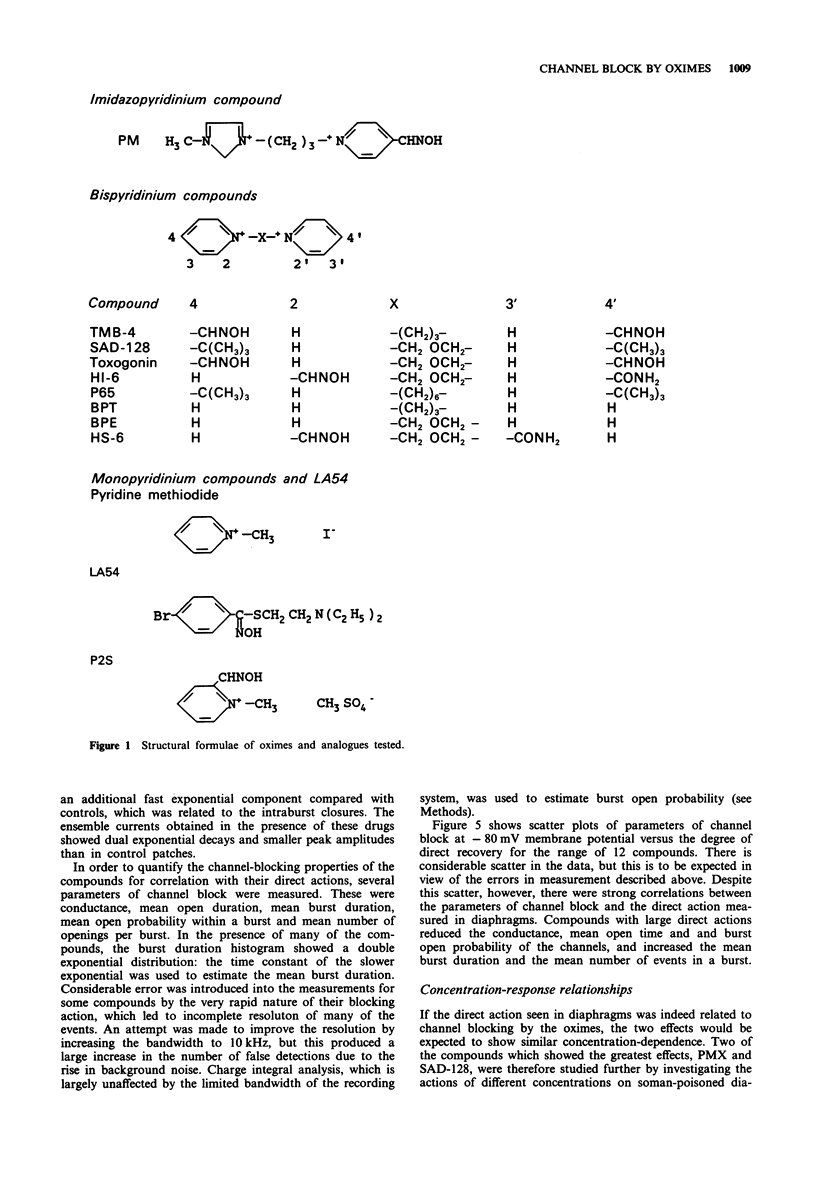
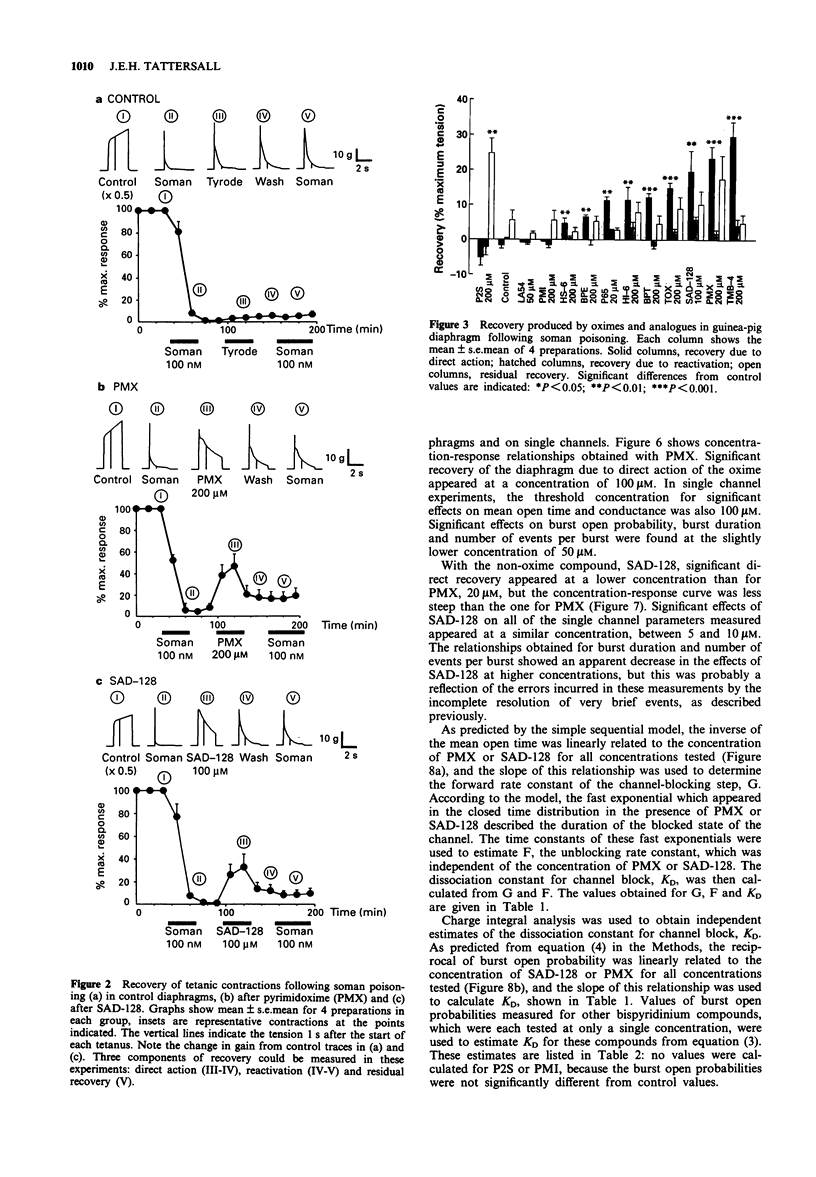
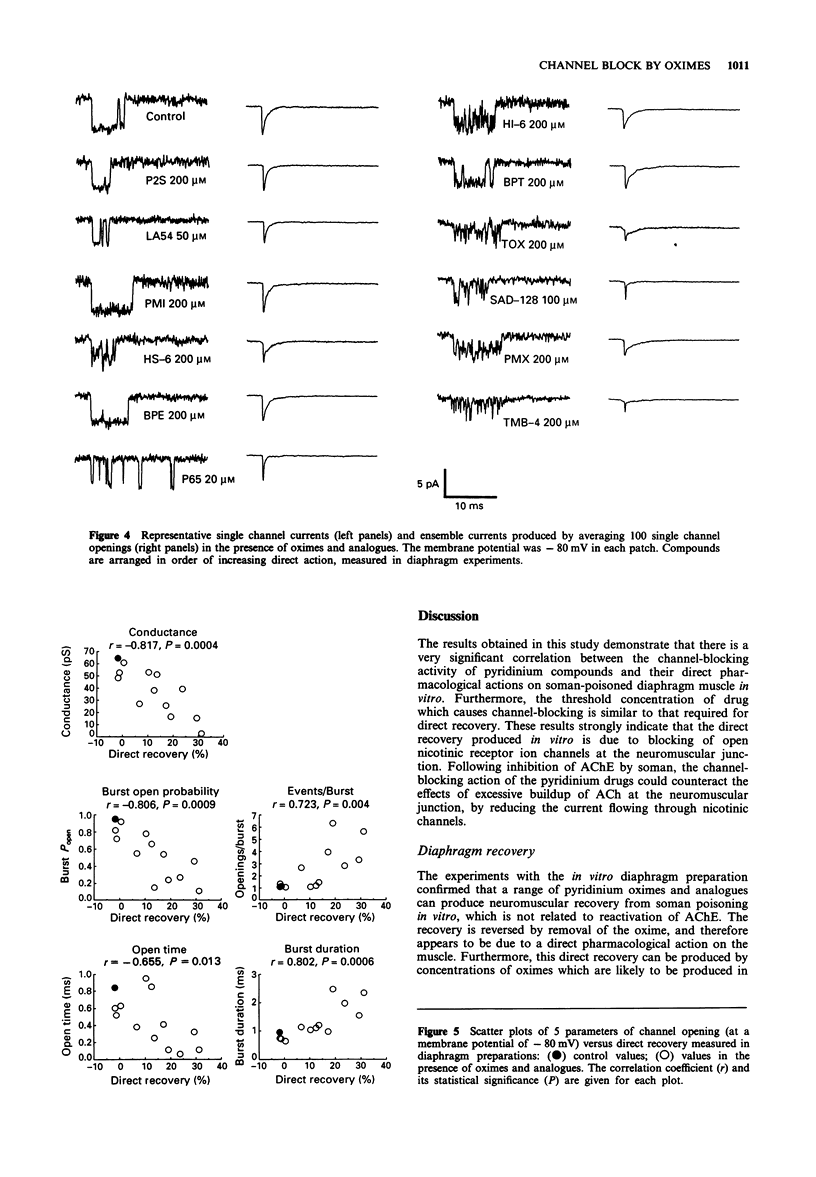
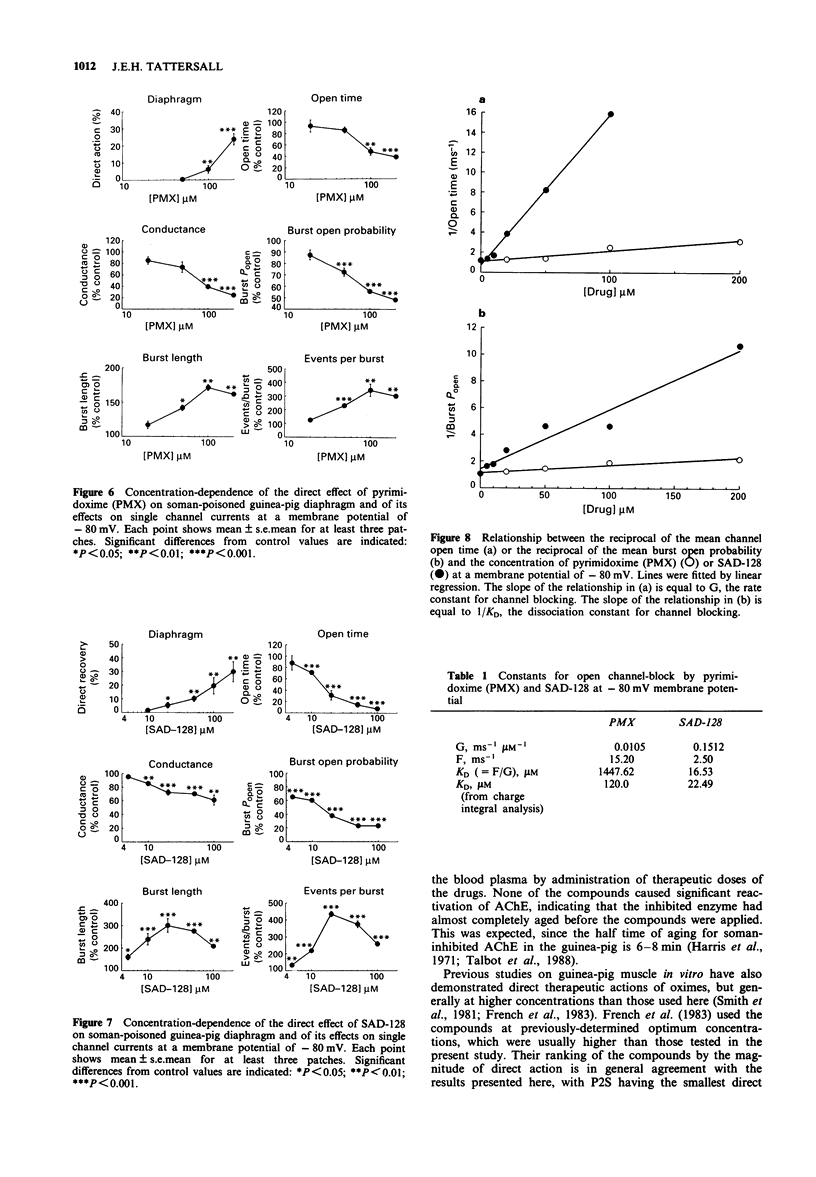



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Albuquerque E. X., Lebeda F. J. Kinetic analysis of end plate currents altered by atropine and scopolamine. Mol Pharmacol. 1978 May;14(3):514–529. [PubMed] [Google Scholar]
- Alkondon M., Albuquerque E. X. The nonoxime bispyridinium compound SAD-128 alters the kinetic properties of the nicotinic acetylcholine receptor ion channel: a possible mechanism for antidotal effects. J Pharmacol Exp Ther. 1989 Sep;250(3):842–852. [PubMed] [Google Scholar]
- Alkondon M., Rao K. S., Albuquerque E. X. Acetylcholinesterase reactivators modify the functional properties of the nicotinic acetylcholine receptor ion channel. J Pharmacol Exp Ther. 1988 May;245(2):543–556. [PubMed] [Google Scholar]
- Brehm P., Kullberg R. Acetylcholine receptor channels on adult mouse skeletal muscle are functionally identical in synaptic and nonsynaptic membrane. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2550–2554. doi: 10.1073/pnas.84.8.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J. G. Toxicology and pharmacology of bispyridium oximes--insight into the mechanism of action vs Soman poisoning in vivo. Fundam Appl Toxicol. 1981 Mar-Apr;1(2):193–202. doi: 10.1016/s0272-0590(81)80058-9. [DOI] [PubMed] [Google Scholar]
- Ecobichon D. J., Comeau A. M., O'Neil W. M., Marshall W. D. Kinetics, distribution, and biotransformation of the chemical HI-6 in the rat, dog, and rhesus monkey. Can J Physiol Pharmacol. 1990 May;68(5):614–621. doi: 10.1139/y90-089. [DOI] [PubMed] [Google Scholar]
- French M. C., Wetherell J. R., White P. D. The reversal by oximes and their de oximinomethyl analogues of neuromuscular block produced by soman. Eur J Pharmacol. 1983 Aug 5;91(4):399–409. doi: 10.1016/0014-2999(83)90164-4. [DOI] [PubMed] [Google Scholar]
- Green M. D., Talbot B. G., Clark C. R. Pharmacokinetics of pralidoxime chloride in the rat. Life Sci. 1986 Dec 8;39(23):2263–2269. doi: 10.1016/0024-3205(86)90405-4. [DOI] [PubMed] [Google Scholar]
- Hamilton M. G., Lundy P. M. HI-6 therapy of soman and tabun poisoning in primates and rodents. Arch Toxicol. 1989;63(2):144–149. doi: 10.1007/BF00316437. [DOI] [PubMed] [Google Scholar]
- Harris L. W., Yamamura H. I., Fleisher J. H. De novo synthesis of acetylcholinesterase in guinea pig retina after inhibition by pinacolyl methylphosphonofluoridate. Biochem Pharmacol. 1971 Oct;20(10):2927–2930. doi: 10.1016/0006-2952(71)90209-7. [DOI] [PubMed] [Google Scholar]
- Inns R. H., Leadbeater L. The efficacy of bispyridinium derivatives in the treatment of organophosphonate poisoning in the guinea-pig. J Pharm Pharmacol. 1983 Jul;35(7):427–433. doi: 10.1111/j.2042-7158.1983.tb04316.x. [DOI] [PubMed] [Google Scholar]
- Klimmek R., Eyer P. Pharmacokinetics and pharmacodynamics of the oxime HI6 in dogs. Arch Toxicol. 1986 Dec;59(4):272–278. doi: 10.1007/BF00290550. [DOI] [PubMed] [Google Scholar]
- Kuhnen-Clausen D. Structure-activity relationship of mono- and bisquaternary pyridines in regard to their parasympatholytic effects. Toxicol Appl Pharmacol. 1972 Nov;23(3):443–454. doi: 10.1016/0041-008x(72)90046-4. [DOI] [PubMed] [Google Scholar]
- LOOMIS T. A., SALAFSKY B. ANTIDOTAL ACTION OF PYRIDINIUM OXIMES IN ANTICHOLINESTERASE POISONING; COMPARATIVE EFFECTS OF SOMAN, SARIN, AND NEOSTIGMINE ON NEUROMUSCULAR FUNCTION. Toxicol Appl Pharmacol. 1963 Nov;5:685–701. doi: 10.1016/0041-008x(63)90062-0. [DOI] [PubMed] [Google Scholar]
- Lundy P. M., Tremblay K. P. Ganglion blocking properties of some bispyridinium soman antagonists. Eur J Pharmacol. 1979 Nov 23;60(1):47–53. doi: 10.1016/0014-2999(79)90051-7. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux C. G., Dua A. K. Pharmacology of HI-6, an H-series oxime. Can J Physiol Pharmacol. 1989 Oct;67(10):1183–1189. doi: 10.1139/y89-188. [DOI] [PubMed] [Google Scholar]
- Smith A. P., Muir A. W. Antidotal action of the oxime HS6 at the soman poisoned neuromuscular junction of the rat and guinea-pig. J Pharm Pharmacol. 1977 Dec;29(12):762–764. doi: 10.1111/j.2042-7158.1977.tb11458.x. [DOI] [PubMed] [Google Scholar]
- Smith A. P., Van der Wiel H. J., Wolthuis O. L. Analysis of oxime-induced neuromuscular recovery in guinea pig, rat and man following soman poisoning in vitro. Eur J Pharmacol. 1981 Mar 26;70(3):371–379. doi: 10.1016/0014-2999(81)90170-9. [DOI] [PubMed] [Google Scholar]
- Su C. T., Tang C. P., Ma C., Shih Y. S., Liu C. Y., Wu M. T. Quantitative structure-activity relationships and possible mechanisms of action of bispyridinium oximes as antidotes against pinacolyl methylphosphonofluoridate. Fundam Appl Toxicol. 1983 Jul-Aug;3(4):271–277. doi: 10.1016/s0272-0590(83)80139-0. [DOI] [PubMed] [Google Scholar]
- Talbot B. G., Anderson D. R., Harris L. W., Yarbrough L. W., Lennox W. J. A comparison of in vivo and in vitro rates of aging of soman-inhibited erythrocyte acetylcholinesterase in different animal species. Drug Chem Toxicol. 1988;11(3):289–305. doi: 10.3109/01480548809017884. [DOI] [PubMed] [Google Scholar]
- Wolthuis O. L., Clason-Van Der Wiel H. J., Visser R. The dependence of the blood level of the oxime HS-6 on the severity of organophosphate poisoning. Eur J Pharmacol. 1976 Oct;39(2):417–421. doi: 10.1016/0014-2999(76)90155-2. [DOI] [PubMed] [Google Scholar]
- Wolthuis O., Vanwersch R. A., Van der Wiel H. J. The efficacy of some bis-pyridinium oximes as antidotes to soman in isolated muscles of several species including man. Eur J Pharmacol. 1981 Mar 26;70(3):355–369. doi: 10.1016/0014-2999(81)90169-2. [DOI] [PubMed] [Google Scholar]
- van Helden H. P., de Lange J., Busker R. W., Melchers B. P. Therapy of organophosphate poisoning in the rat by direct effects of oximes unrelated to ChE reactivation. Arch Toxicol. 1991;65(7):586–593. doi: 10.1007/BF01973721. [DOI] [PubMed] [Google Scholar]



