Abstract
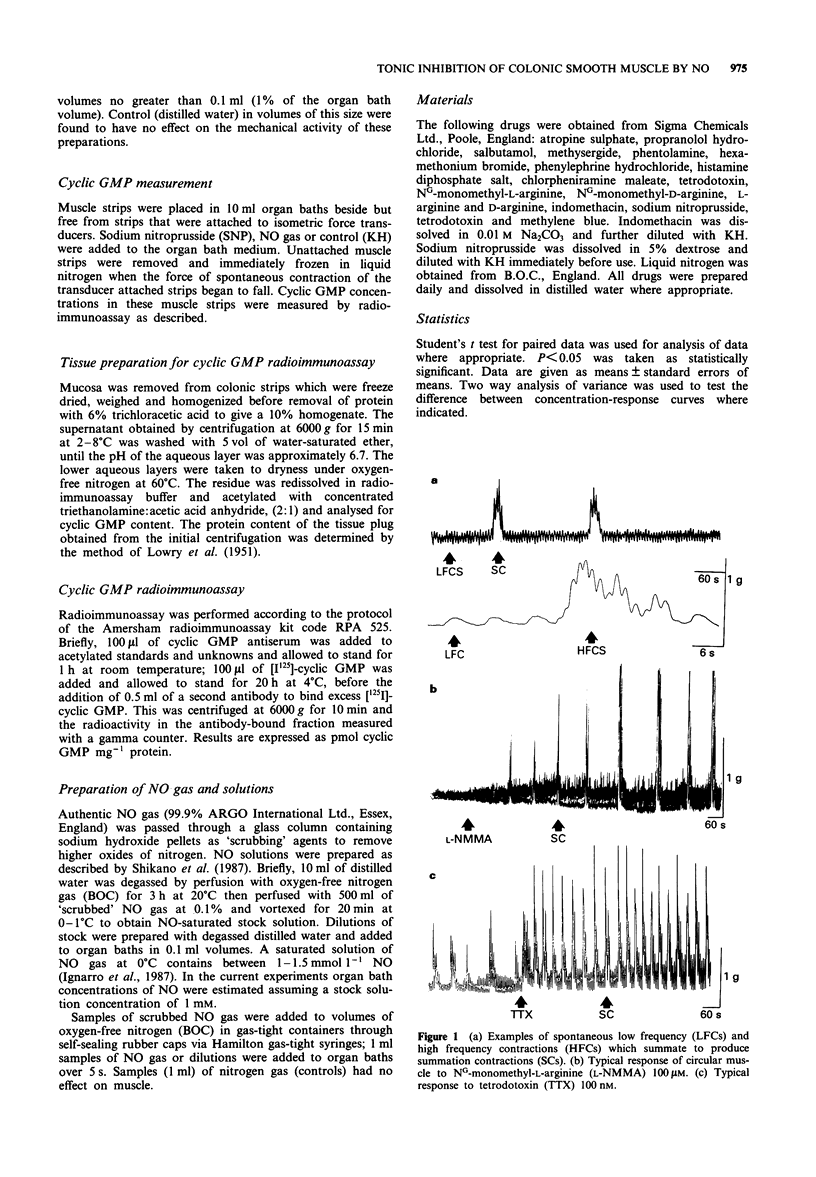
1 The role of the L-arginine-nitric oxide (NO) pathway in tonic neural inhibition of spontaneous mechanical activity of distal colonic circular smooth muscle (DCCSM) was investigated in male Wistar rats. 2 Muscle strips were mounted in organ baths and spontaneous contractions recorded with isometric force transducers. They were characterized as low frequency (LFCs) 0.41 +/- 0.03 N cm-2 or high frequency contractions (HFCs) 0.22 +/- 0.04 N cm-2. The latter occurred intermittently to produce summation contractions (SCs) range 0.5-12 N cm-2. 3 Tetrodotoxin (100 nM) increased the forces of LFCs and SCs. Increase in force to tetrodotoxin did not occur after incubation of the muscle with NG-monomethyl-L-arginine (L-NMMA) 500 microM, an inhibitor of NO biosynthesis. 4 L-NMMA but not its enantiomer D-NMMA increased the force of LFCs (EC50: 200 microM) and SCS (EC50:175 microM) in a concentration-dependent manner which was reversed by L-arginine but not by D-arginine. 5 Muscle, precontracted by acetylcholine, relaxed to sodium nitroprusside (EC50:1.8 microM) NO gas (EV50:70 microliters) and NO solutions (EC50:4 microM) in a concentration-dependent manner. Guanosine 3':5'-cyclic monophosphate tissue concentrations (pmol mg-1 protein) were elevated in muscle after relaxation by sodium nitroprusside (500 microM) from 0.32 +/- 0.06 to 1.2 +/- 0.37 and by 1 ml of NO gas from 0.49 +/- 0.05 to 1.54 +/- 0.14. 6 These data suggest that DCCSM is under tonic neural inhibition mediated by NO biosynthesis.
Full text
PDF
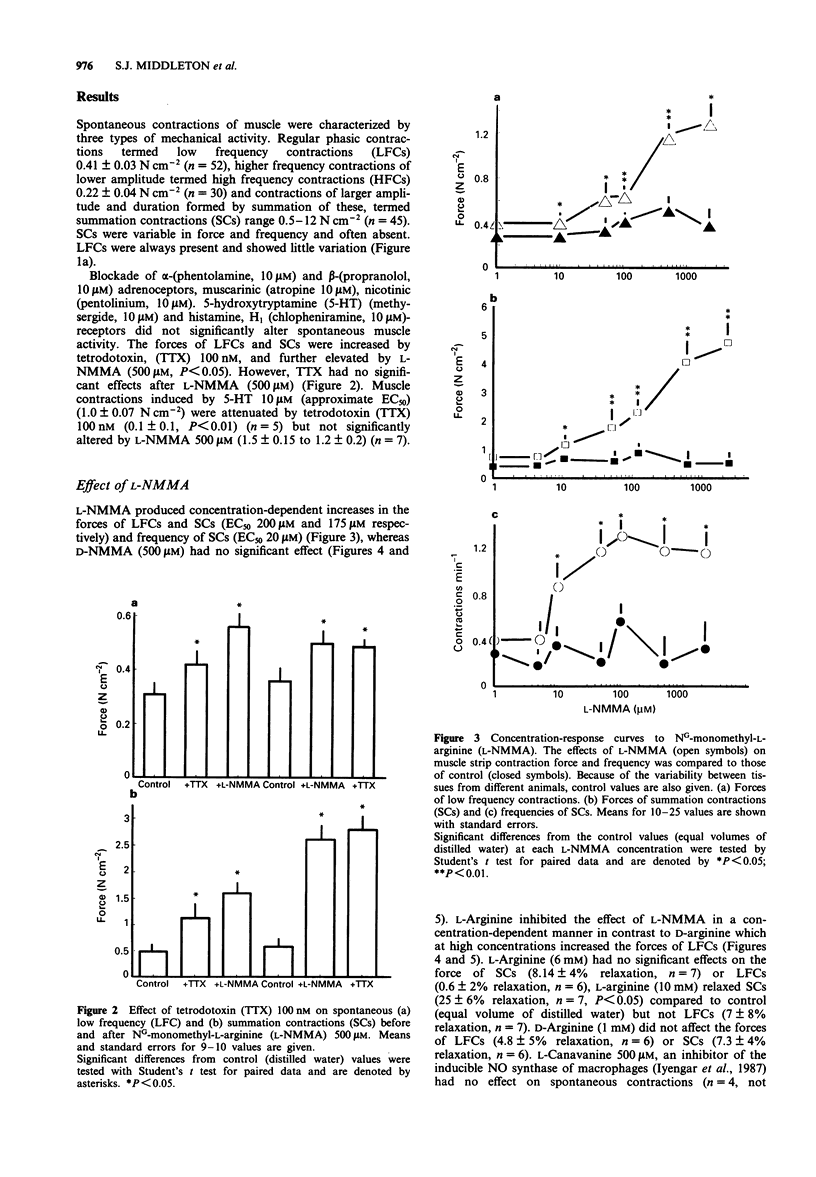
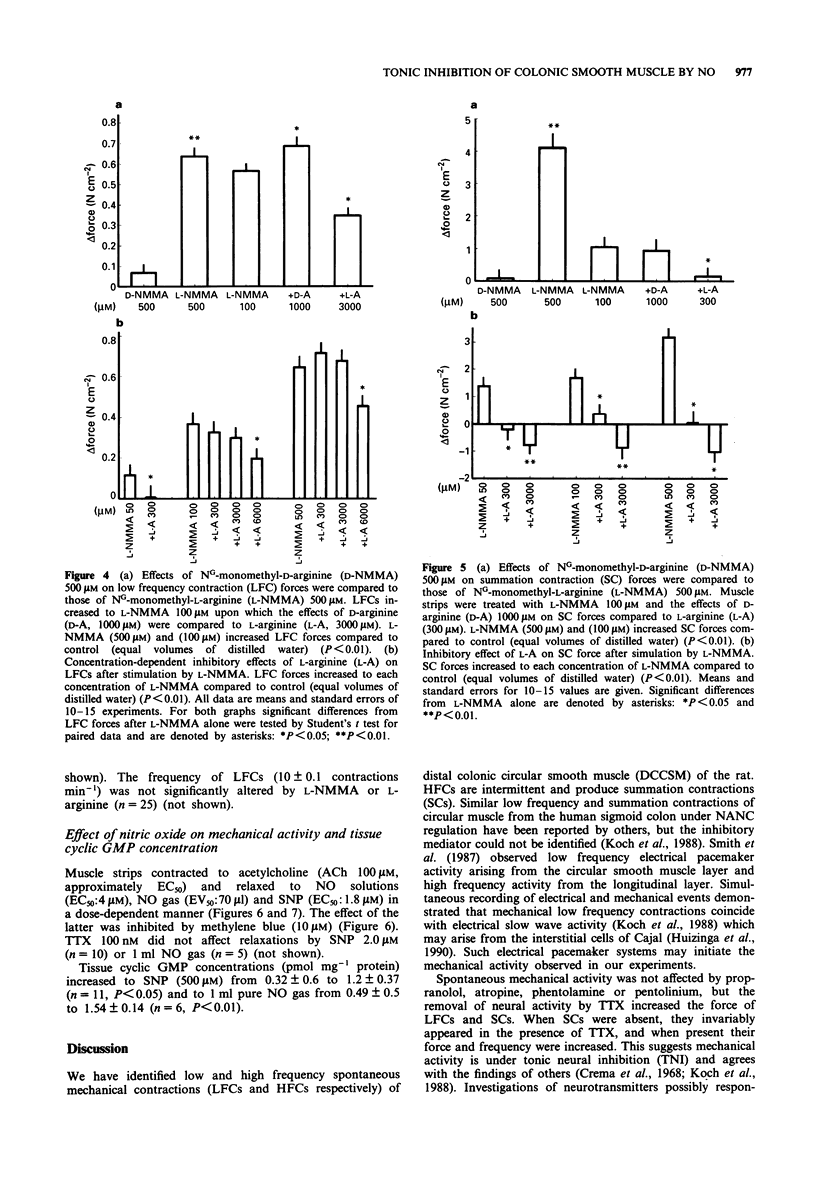

Selected References
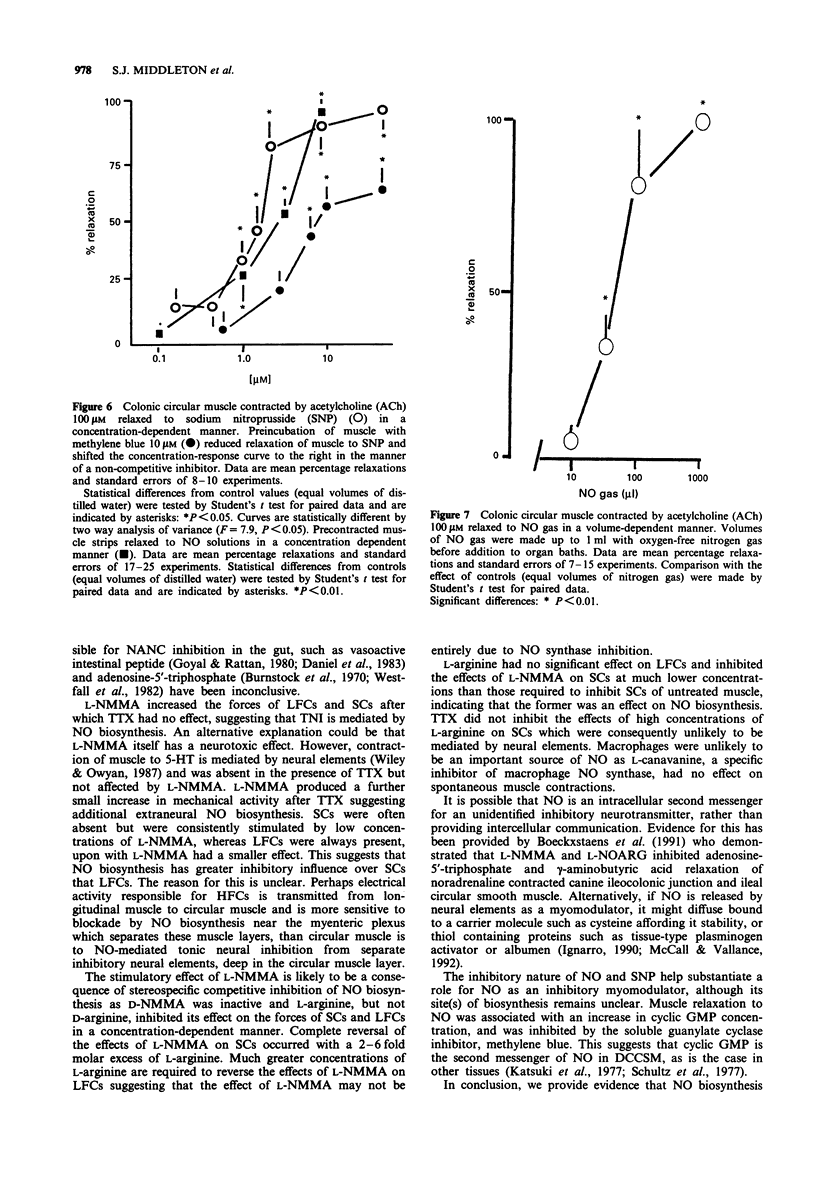
These references are in PubMed. This may not be the complete list of references from this article.
- Boeckxstaens G. E., Pelckmans P. A., Bult H., De Man J. G., Herman A. G., Van Maercke Y. M. Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur J Pharmacol. 1990 Nov 6;190(1-2):239–246. doi: 10.1016/0014-2999(90)94132-h. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Bult H., De Man J. G., Herman A. G., van Maercke Y. M. Evidence for nitric oxide as mediator of non-adrenergic non-cholinergic relaxations induced by ATP and GABA in the canine gut. Br J Pharmacol. 1991 Feb;102(2):434–438. doi: 10.1111/j.1476-5381.1991.tb12191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Burleigh D. E. Ng-nitro-L-arginine reduces nonadrenergic, noncholinergic relaxations of human gut. Gastroenterology. 1992 Feb;102(2):679–683. doi: 10.1016/0016-5085(92)90120-n. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Kao H. W., Tan S. T., Lechago J., Snape W. J., Jr Effect of acute experimental colitis on rabbit colonic smooth muscle. Am J Physiol. 1986 Oct;251(4 Pt 1):G538–G545. doi: 10.1152/ajpgi.1986.251.4.G538. [DOI] [PubMed] [Google Scholar]
- Crema A., Del Tacca M., Frigo G. M., Lecchini S. Presence of a non-adrenergic inhibitory system in the human colon. Gut. 1968 Dec;9(6):633–637. doi: 10.1136/gut.9.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel H. H., Thornbury K. D., Ward S. M., Sanders K. M. Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol. 1991 May;260(5 Pt 1):G789–G792. doi: 10.1152/ajpgi.1991.260.5.G789. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., Helmy-Elkholy A., Jager L. P., Kannan M. S. Neither a purine nor VIP is the mediator of inhibitory nerves of opossum oesophageal smooth muscle. J Physiol. 1983 Mar;336:243–260. doi: 10.1113/jphysiol.1983.sp014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Gordon A. R., Siegman M. J. Mechanical properties of smooth muscle. I. Length-tension and force-velocity relations. Am J Physiol. 1971 Nov;221(5):1243–1249. doi: 10.1152/ajplegacy.1971.221.5.1243. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S., Said S. I. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 1980 Nov 27;288(5789):378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- Hata F., Ishii T., Kanada A., Yamano N., Kataoka T., Takeuchi T., Yagasaki O. Essential role of nitric oxide in descending inhibition in the rat proximal colon. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1400–1406. doi: 10.1016/0006-291x(90)91605-r. [DOI] [PubMed] [Google Scholar]
- Huizinga J. D., Berezin I., Daniel E. E., Chow E. Inhibitory innervation of colonic smooth muscle cells and interstitial cells of Cajal. Can J Physiol Pharmacol. 1990 Mar;68(3):447–454. doi: 10.1139/y90-063. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990 Nov;16(5):477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S., Arnold W. P., Murad F. Effects of sodium nitroprusside, nitroglycerin, and sodium azide on levels of cyclic nucleotides and mechanical activity of various tissues. J Cyclic Nucleotide Res. 1977 Aug;3(4):239–247. [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Kinetic characteristics of nitric oxide synthase from rat brain. Biochem J. 1990 Jul 1;269(1):207–210. doi: 10.1042/bj2690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T. R., Carney J. A., Go V. L., Szurszewski J. H. Spontaneous contractions and some electrophysiologic properties of circular muscle from normal sigmoid colon and ulcerative colitis. Gastroenterology. 1988 Jul;95(1):77–84. doi: 10.1016/0016-5085(88)90293-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCall T., Vallance P. Nitric oxide takes centre-stage with newly defined roles. Trends Pharmacol Sci. 1992 Jan;13(1):1–6. doi: 10.1016/0165-6147(92)90002-n. [DOI] [PubMed] [Google Scholar]
- Merlo A., Cohen S. Neuropeptide responses and mechanics of the proximal and distal feline colon in vitro. Am J Physiol. 1988 Dec;255(6 Pt 1):G787–G793. doi: 10.1152/ajpgi.1988.255.6.G787. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu K., Diamond J. Role of cGMP in relaxation of vascular and other smooth muscle. Can J Physiol Pharmacol. 1989 Apr;67(4):251–262. doi: 10.1139/y89-042. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K., Schultz K., Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic GMP levels in rat ductus deferens. Nature. 1977 Feb 24;265(5596):750–751. doi: 10.1038/265750a0. [DOI] [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. K., Reed J. B., Sanders K. M. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol. 1987 Mar;252(3 Pt 1):C290–C299. doi: 10.1152/ajpcell.1987.252.3.C290. [DOI] [PubMed] [Google Scholar]
- Tøttrup A., Glavind E. B., Svane D. Involvement of the L-arginine-nitric oxide pathway in internal anal sphincter relaxation. Gastroenterology. 1992 Feb;102(2):409–415. doi: 10.1016/0016-5085(92)90084-c. [DOI] [PubMed] [Google Scholar]
- Westfall D. P., Hogaboom G. K., Colby J., O'Donnell J. P., Fedan J. S. Direct evidence against a role of ATP as the nonadrenergic, noncholinergic inhibitory neurotransmitter in guinea pig tenia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7041–7045. doi: 10.1073/pnas.79.22.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J., Owyang C. Participation of serotonin and substance P in the action of cholecystokinin on colonic motility. Am J Physiol. 1987 Mar;252(3 Pt 1):G431–G435. doi: 10.1152/ajpgi.1987.252.3.G431. [DOI] [PubMed] [Google Scholar]



