Abstract
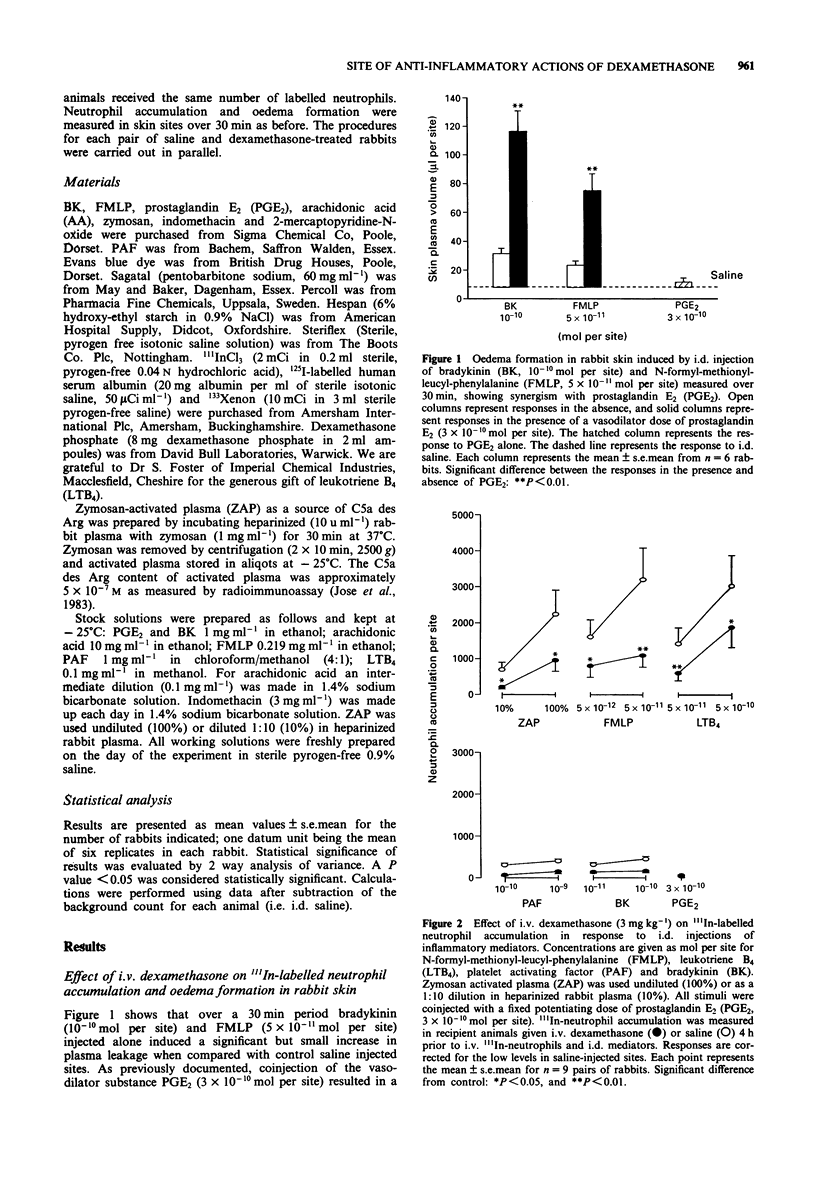
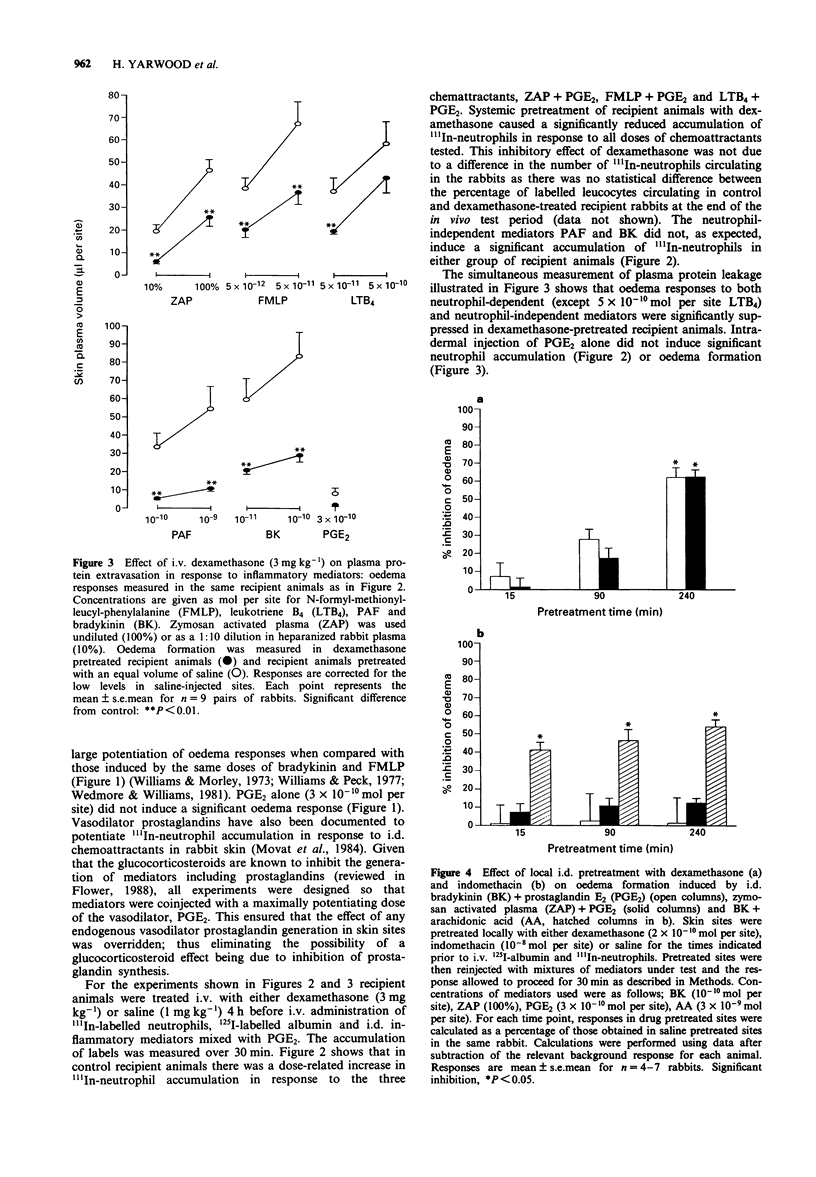
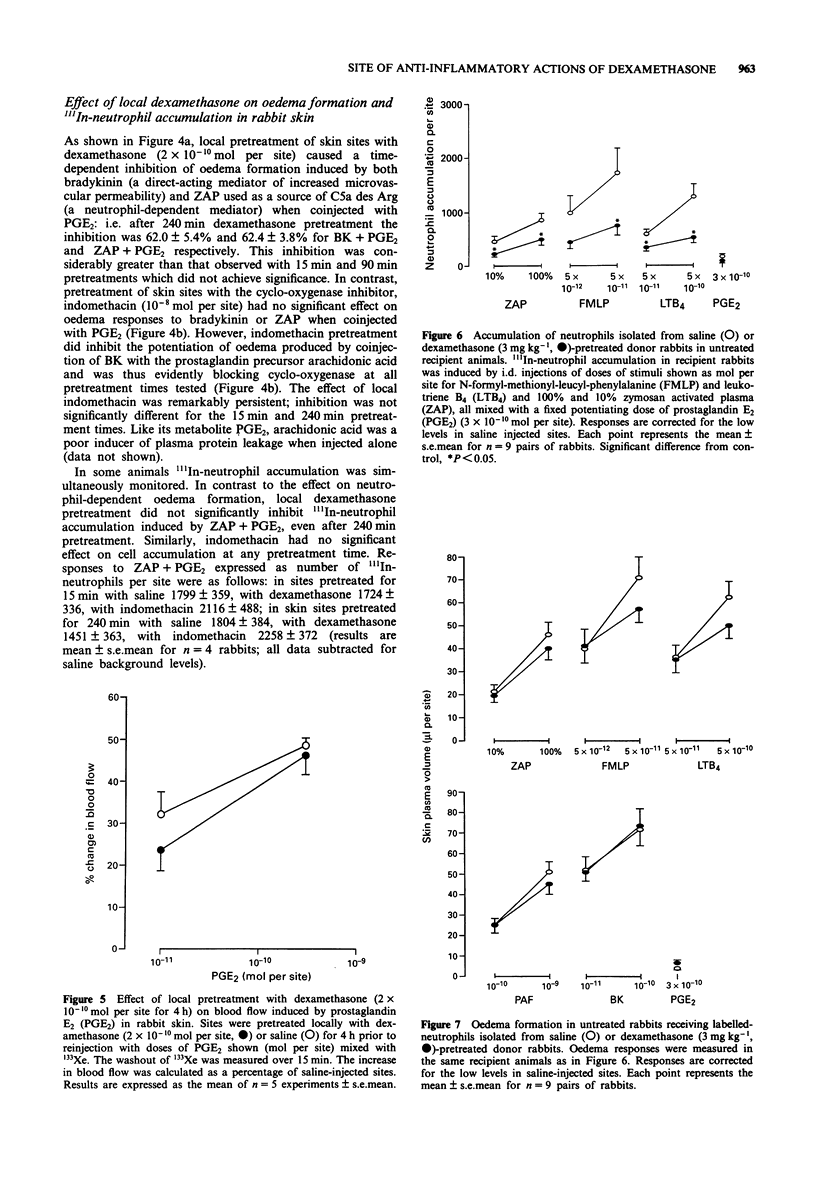
1. The anti-inflammatory actions of dexamethasone on vascular and leukocyte responses in rabbit skin were investigated. 2. Neutrophil accumulation and oedema formation were simultaneously measured as the local accumulation of i.v. administered 111In-labelled neutrophils and 125I-labelled albumin. Systemically administered dexamethasone (3 mg kg-1) inhibited neutrophil accumulation induced by i.d. zymosan activated plasma (ZAP), N-formyl-methionyl-leucyl-phenylalanine (FMLP) and leukotriene B4 (LTB4) when co-injected with prostaglandin E2 (PGE2). Dexamethasone also inhibited oedema formation elicited by these stimuli and the responses induced by i.d. platelet activating factor (PAF)+PGE2 and bradykinin (BK)+PGE2. 3. Intradermal dexamethasone (2 x 10(-10) mol per site) but not indomethacin (10(-8) mol per site) inhibited oedema formation induced by i.d. ZAP+PGE2 and BK+PGE2. This inhibitory effect of dexamethasone was significant only with pretreatment periods of 4 h, shorter pretreatment periods resulting in greatly reduced effects. Intradermal dexamethasone had no effect on neutrophil accumulation induced by ZAP+PGE2. 4. Intradermal dexamethasone (2 x 10(-10) mol per site) had no effect on increase in blood flow induced by PGE2 as measured by 133Xenon clearance. 5. The accumulation of neutrophils isolated from donor rabbits pretreated with i.v. saline or dexamethasone (3 mg kg-1) was investigated in untreated recipient rabbits. The accumulation of neutrophils, induced by ZAP+PGE2, FMLP+PGE2 and LTB4+PGE2, from dexamethasone-pretreated donors was significantly smaller than the accumulation of neutrophils from saline-pretreated donors. 6. The results of this study suggest that dexamethasone can have a direct effect on vascular endothelial cells resulting in an inhibition of oedema formation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATHENS J. W., HAAB O. P., RAAB S. O., MAUER A. M., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961 Jun;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk J., Goldschmidt T., Smedegård G., Arfors K. E. Methylprednisolone acts at the endothelial cell level reducing inflammatory responses. Acta Physiol Scand. 1985 Feb;123(2):221–224. doi: 10.1111/j.1748-1716.1985.tb07581.x. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Carnuccio R., Di Rosa M., Guerrasio B., Iuvone T., Sautebin L. Vasocortin: a novel glucocorticoid-induced anti-inflammatory protein. Br J Pharmacol. 1987 Mar;90(3):443–445. doi: 10.1111/j.1476-5381.1987.tb11193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church M. K., Miller P. Time courses of the anti-anaphylactic and anti-inflammatory effects of dexamethasone in the rat and mouse. Br J Pharmacol. 1978 Apr;62(4):481–486. doi: 10.1111/j.1476-5381.1978.tb07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino G., Flower R. J., Browning J. L., Sinclair L. K., Pepinsky R. B. Recombinant human lipocortin 1 inhibits thromboxane release from guinea-pig isolated perfused lung. Nature. 1987 Jul 16;328(6127):270–272. doi: 10.1038/328270a0. [DOI] [PubMed] [Google Scholar]
- Cirino G., Flower R. J. Human recombinant lipocortin 1 inhibits prostacyclin production by human umbilical artery in vitro. Prostaglandins. 1987 Jul;34(1):59–62. doi: 10.1016/0090-6980(87)90262-0. [DOI] [PubMed] [Google Scholar]
- Cirino G., Peers S. H., Flower R. J., Browning J. L., Pepinsky R. B. Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci U S A. 1989 May;86(9):3428–3432. doi: 10.1073/pnas.86.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloix J. F., Colard O., Rothhut B., Russo-Marie F. Characterization and partial purification of 'renocortins': two polypeptides formed in renal cells causing the anti-phospholipase-like action of glucocorticoids. Br J Pharmacol. 1983 May;79(1):313–321. doi: 10.1111/j.1476-5381.1983.tb10526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Dedman J. R. Protein terminology tangle. Nature. 1990 May 17;345(6272):212–212. doi: 10.1038/345212a0. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Calignano A., Carnuccio R., Ialenti A., Sautebin L. Multiple control of inflammation by glucocorticoids. Agents Actions. 1986 Jan;17(3-4):284–289. doi: 10.1007/BF01982621. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Flower R. J., Hirata F., Parente L., Russo-Marie F. Anti-phospholipase proteins. Prostaglandins. 1984 Oct;28(4):441–442. doi: 10.1016/0090-6980(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Errasfa M., Russo-Marie F. A purified lipocortin shares the anti-inflammatory effect of glucocorticosteroids in vivo in mice. Br J Pharmacol. 1989 Aug;97(4):1051–1058. doi: 10.1111/j.1476-5381.1989.tb12561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R. J. Eleventh Gaddum memorial lecture. Lipocortin and the mechanism of action of the glucocorticoids. Br J Pharmacol. 1988 Aug;94(4):987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster S. J., McCormick M. E. The mechanism of the anti-inflammatory activity of glucocorticosteroids. Agents Actions. 1985 Mar;16(1-2):58–59. doi: 10.1007/BF01999649. [DOI] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose P. J., Forrest M. J., Williams T. J. Detection of the complement fragment C5a in inflammatory exudates from the rabbit peritoneal cavity using radioimmunoassay. J Exp Med. 1983 Dec 1;158(6):2177–2182. doi: 10.1084/jem.158.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidbey K. H., Kligman A. M. Assay of topical corticosteroids by suppression of experimental inflammation in humans. J Invest Dermatol. 1974 Sep;63(3):292–297. doi: 10.1111/1523-1747.ep12680178. [DOI] [PubMed] [Google Scholar]
- Katori M., Oda T., Nagai K. A site of action of dexamethasone on leukocyte extravasation in microcirculation. Agents Actions. 1990 Jan;29(1-2):24–26. doi: 10.1007/BF01964709. [DOI] [PubMed] [Google Scholar]
- Lorant D. E., Patel K. D., McIntyre T. M., McEver R. P., Prescott S. M., Zimmerman G. A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991 Oct;115(1):223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R. The effect of anti-inflammatory agents and inflammation on granulocyte adherence. Evidence for regulation by plasma factors. Am J Med. 1976 Nov;61(5):597–607. doi: 10.1016/0002-9343(76)90137-6. [DOI] [PubMed] [Google Scholar]
- Majeski J. A., Alexander J. W. The steroid effect on the in vitro human neutrophil chemotactic response. J Surg Res. 1976 Oct;21(4):265–268. doi: 10.1016/0022-4804(76)90036-6. [DOI] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks R., Sawyer M. Glucocorticoid-induced vasoconstriction in human skin. An inhibitory role on phospholipase A2 activity. Arch Dermatol. 1986 Aug;122(8):881–883. [PubMed] [Google Scholar]
- McEver R. P., Beckstead J. H., Moore K. L., Marshall-Carlson L., Bainton D. F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989 Jul;84(1):92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movat H. Z., Rettl C., Burrowes C. E., Johnston M. G. The in vivo effect of leukotriene B4 on polymorphonuclear leukocytes and the microcirculation. Comparison with activated complement (C5a des Arg) and enhancement by prostaglandin E2. Am J Pathol. 1984 May;115(2):233–244. [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S., Rampart M., Hellewell P. G., Jose P. J., Harlan J. M., Edwards A. J., Williams T. J. Accumulation of 111In-neutrophils in rabbit skin in allergic and non-allergic inflammatory reactions in vivo. Inhibition by neutrophil pretreatment in vitro with a monoclonal antibody recognizing the CD18 antigen. J Immunol. 1989 May 1;142(9):3193–3198. [PubMed] [Google Scholar]
- Osterrieder W. 9-Amino-1,2,3,4-tetrahydroacridine (THA) is a potent blocker of cardiac potassium channels. Br J Pharmacol. 1987 Nov;92(3):521–525. doi: 10.1111/j.1476-5381.1987.tb11352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers S. H., Flower R. J. Site of anti-inflammatory action of dexamethasone in rabbit skin. Eur J Pharmacol. 1991 Apr 10;196(1):37–41. doi: 10.1016/0014-2999(91)90406-g. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Evidence that neutrophil accumulation induced by interleukin-1 requires both local protein biosynthesis and neutrophil CD18 antigen expression in vivo. Br J Pharmacol. 1988 Aug;94(4):1143–1148. doi: 10.1111/j.1476-5381.1988.tb11632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J., Brookes D. B. Topical corticosteroids--an experimental evaluation of the vasoconstrictor test as an index of anti-inflammatory activity. Br J Dermatol. 1968 May;80(5):328–336. doi: 10.1111/j.1365-2133.1968.tb12307.x. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., Freeland H. S., Peters S. P., Brown K. E., Derse C. P. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther. 1989 Aug;250(2):598–605. [PubMed] [Google Scholar]
- Tsurufuji S., Kurihara A., Ojima F. Mechanisms of anti-inflammatory action of dexamethasone: blockade by hydrocortisone mesylate and actinomycin D of the inhibitory effect of dexamethasone on leukocyte infiltration in inflammatory sites. J Pharmacol Exp Ther. 1984 Apr;229(1):237–243. [PubMed] [Google Scholar]
- Tsurufuji S., Sugio K., Takemasa F. The role of glucocorticoid receptor and gene expression in the anti-inflammatory action of dexamethasone. Nature. 1979 Aug 2;280(5721):408–410. doi: 10.1038/280408a0. [DOI] [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Yagi M., Omata M., Hirasawa N., Mue S., Tsurufuji S., Ohuchi K. Stimulation of neutrophil adherence to vascular endothelial cells by histamine and thrombin and its inhibition by PAF antagonists and dexamethasone. Br J Pharmacol. 1991 Jan;102(1):239–245. doi: 10.1111/j.1476-5381.1991.tb12160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Goldstein I., Hoffstein S. Prostaglandins and the modulation by cyclic nucleotides of lysosomal enzyme release. Adv Prostaglandin Thromboxane Res. 1976;2:803–814. [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Peck M. J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- Williams T. J. Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br J Pharmacol. 1979 Mar;65(3):517–524. doi: 10.1111/j.1476-5381.1979.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. J., Yarwood H. Effect of glucocorticosteroids on microvascular permeability. Am Rev Respir Dis. 1990 Feb;141(2 Pt 2):S39–S43. [PubMed] [Google Scholar]



