Abstract
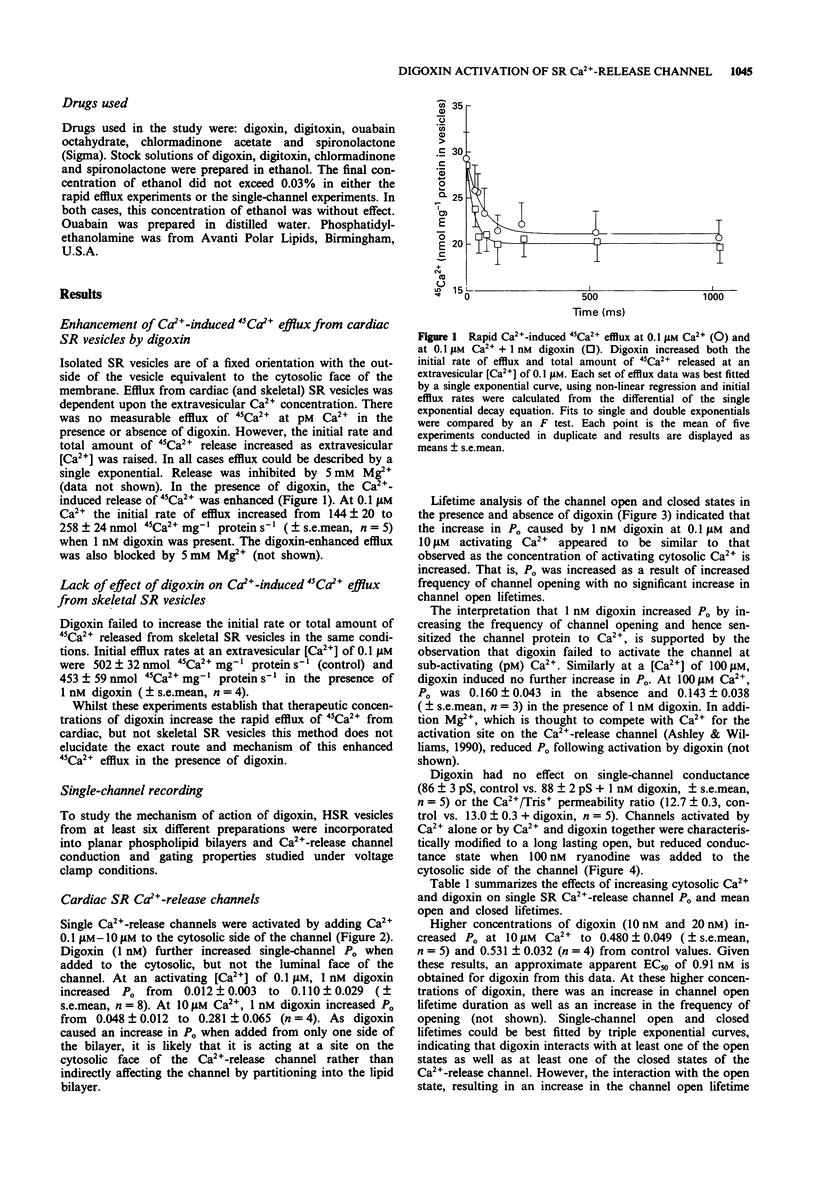
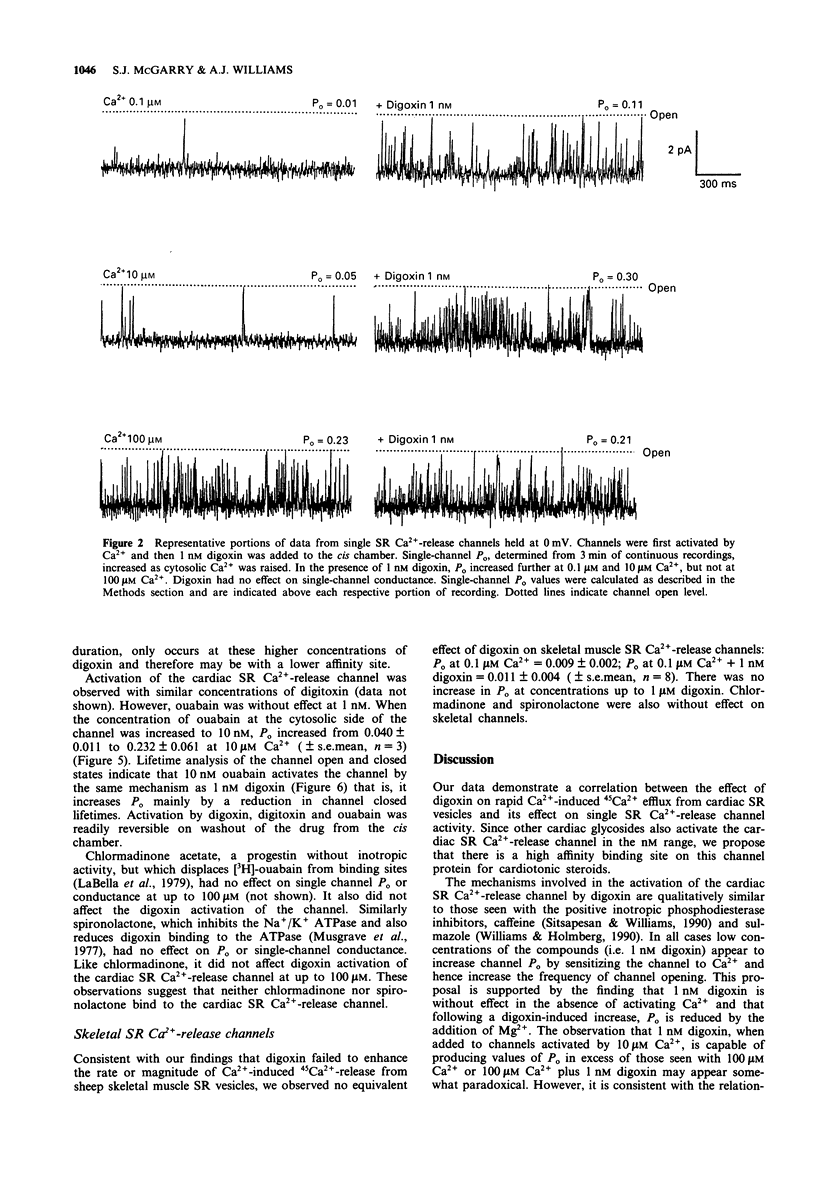
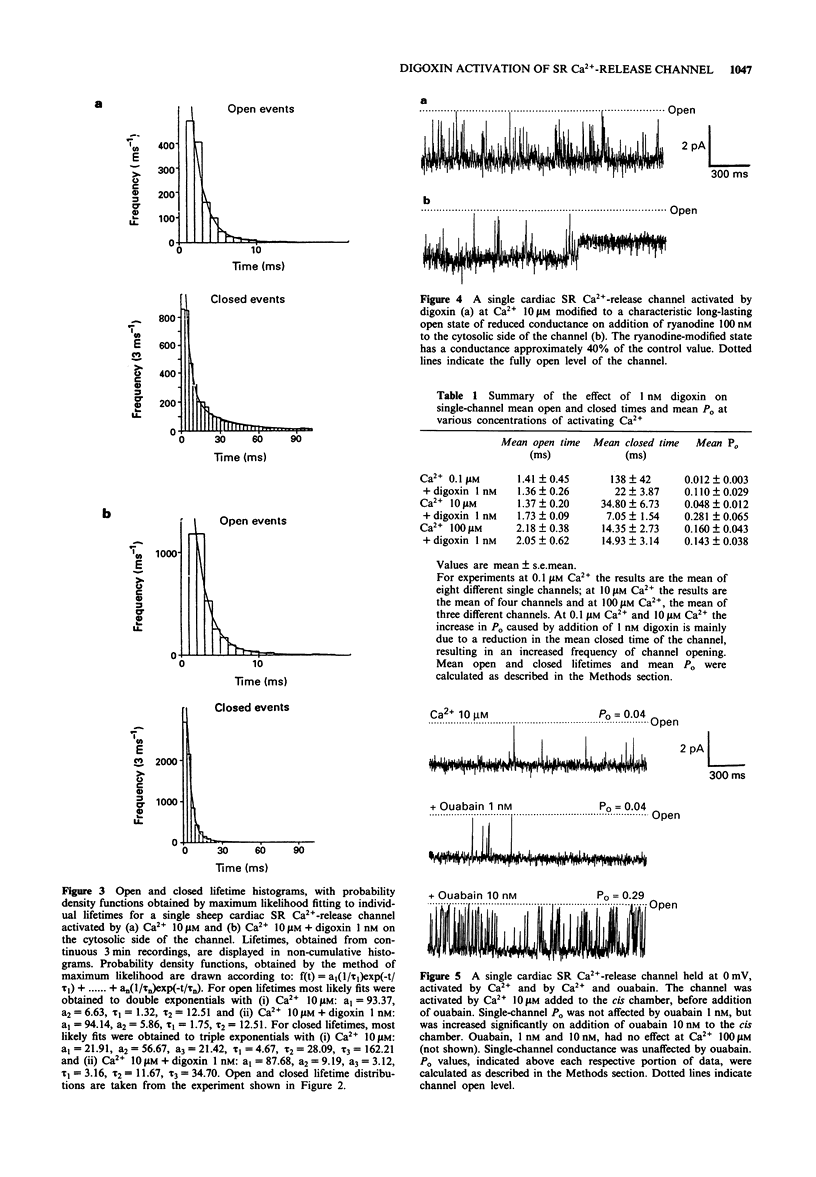
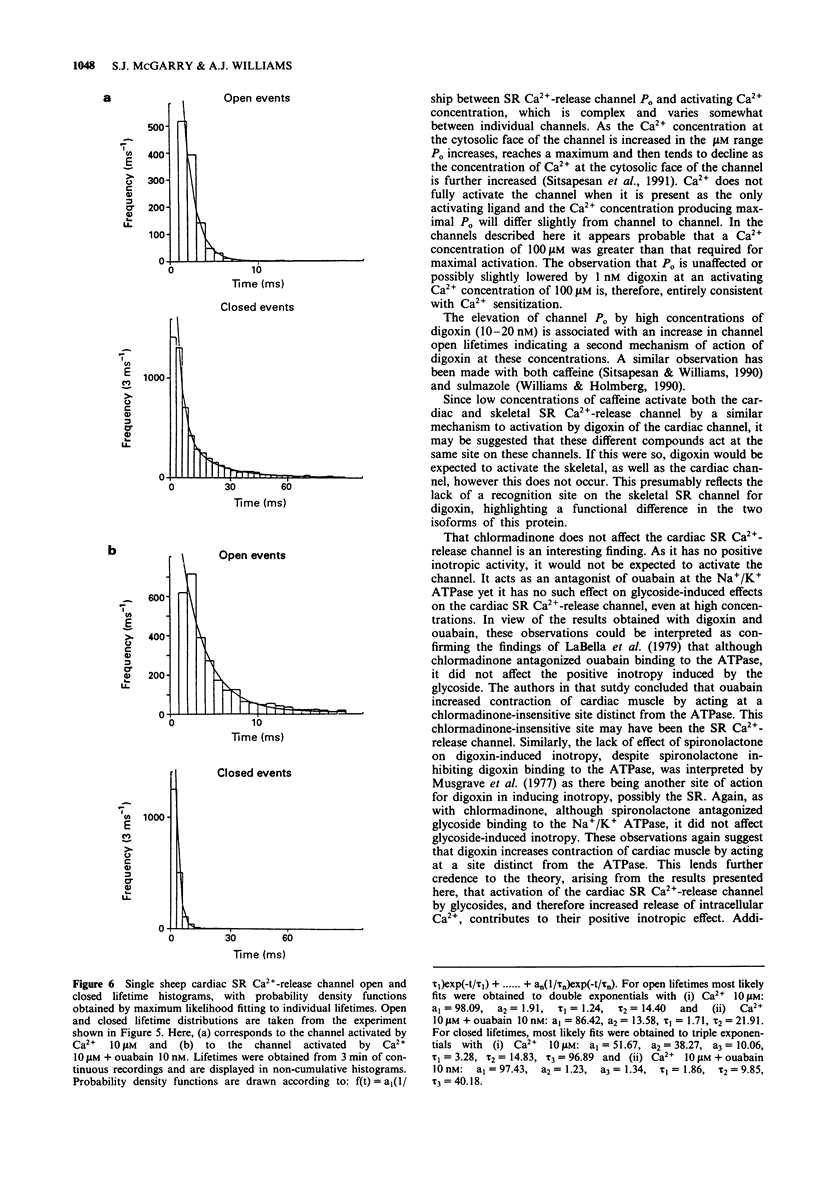
1. The effect of digoxin on rapid 45Ca2+ efflux from cardiac and skeletal sarcoplasmic reticulum (SR) vesicles was investigated. Additionally the interaction of digoxin with single cardiac and skeletal muscle SR Ca(2+)-release channels incorporated into planar phospholipid bilayers and held under voltage clamp was determined. 2. Digoxin (1 nM) increased the initial rate and amount of Ca(2+)-induced release of 45Ca2+ from cardiac SR vesicles, passively loaded with 45CaCl2, at an extravesicular [Ca2+] of 0.1 microM. The efflux in the presence and absence of digoxin was inhibited at pM extravesicular Ca2+ and blocked by 5 mM Mg2+. 3. To elucidate the mechanism of action of digoxin, single-channel recording was used. Digoxin (1-20 nM) increased single-channel open probability (Po) when added to the cytosolic but not the luminal face of the cardiac channel in the presence of sub-maximally activating Ca2+ (0.1 microM-10 microM) with an EC50 of 0.91 nM at 10 microM Ca2+. The mechanisms underlying the action of digoxin appear to be concentration-dependent. The activation observed at 1 nM digoxin appears to be consistent with the sensitization of the channel to the effects of Ca2+. At higher concentrations the drug appears to interact synergistically with Ca2+ to produce values of Po considerably greater than those seen with Ca2+ as the sole activating ligand. 4. Digoxin had no effect on single-channel conductance or the Ca2+/Tris permeability ratio. In channels activated by digoxin the Po was decreased by Mg2+.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Orchard C. H. Factors influencing free intracellular calcium concentration in quiescent ferret ventricular muscle. J Physiol. 1984 May;350:615–630. doi: 10.1113/jphysiol.1984.sp015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Eisner D. A., Pirolo J. S., Smith G. L. The relationship between intracellular calcium and contraction in calcium-overloaded ferret papillary muscles. J Physiol. 1985 Jul;364:169–182. doi: 10.1113/jphysiol.1985.sp015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley R. H., Williams A. J. Divalent cation activation and inhibition of single calcium release channels from sheep cardiac sarcoplasmic reticulum. J Gen Physiol. 1990 May;95(5):981–1005. doi: 10.1085/jgp.95.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Bridge J. H. Effect of acetylstrophanthidin on twitches, microscopic tension fluctuations and cooling contractures in rabbit ventricle. J Physiol. 1988 Oct;404:53–69. doi: 10.1113/jphysiol.1988.sp017278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Hart G., Levi A. J. Dissociation between force and intracellular sodium activity with strophanthidin in isolated sheep Purkinje fibres. J Physiol. 1986 Dec;381:311–331. doi: 10.1113/jphysiol.1986.sp016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. An analysis of the actions of low concentrations of ouabain on membrane currents in Purkinje fibres. J Physiol. 1976 Aug;260(1):75–103. doi: 10.1113/jphysiol.1976.sp011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont Y. A rapid-filtration technique for membrane fragments or immobilized enzymes: measurements of substrate binding or ion fluxes with a few-millisecond time resolution. Anal Biochem. 1984 Nov 1;142(2):504–510. doi: 10.1016/0003-2697(84)90496-2. [DOI] [PubMed] [Google Scholar]
- Dutta S., Goswami S., Datta D. K., Lindower J. O., Marks B. H. The uptake and binding of six radiolabeled cardiac glycosides by guinea-pig hearts and by isolated sarcoplasmic reticulum. J Pharmacol Exp Ther. 1968 Nov;164(1):10–21. [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. The role of the sodium pump in the effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:279–301. doi: 10.1113/jphysiol.1979.sp012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A. The Wellcome prize lecture. Intracellular sodium in cardiac muscle: effects on contraction. Exp Physiol. 1990 Jul;75(4):437–457. doi: 10.1113/expphysiol.1990.sp003422. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke U., Klaus W. Sodium-dependent cardiac glycoside binding: experimental evidence and hypothesis. Br J Pharmacol. 1978 Feb;62(2):255–257. doi: 10.1111/j.1476-5381.1978.tb08453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysel-Burton J., Godfraind T. Low-potassium or ouabain inotropy in cardiac muscle [proceedings]. J Physiol. 1979 Oct;295:52P–53P. [PubMed] [Google Scholar]
- Godfraind T., Ghysel-Burton J. Binding sites related to ouabain-induced stimulation or inhibition of the sodium pump. Nature. 1977 Jan 13;265(5590):165–166. doi: 10.1038/265165a0. [DOI] [PubMed] [Google Scholar]
- Hart G., Noble D., Shimoni Y. The effects of low concentrations of cardiotonic steroids on membrane currents and tension in sheep Purkinje fibres. J Physiol. 1983 Jan;334:103–131. doi: 10.1113/jphysiol.1983.sp014483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBella F. S., Bihler I., Kim R. S. Progesterone derivative binds to cardiac ouabain receptor and shows dissociation between sodium pump inhibition and increased contractile force. Nature. 1979 Apr 5;278(5704):571–573. doi: 10.1038/278571a0. [DOI] [PubMed] [Google Scholar]
- Marban E., Tsien R. W. Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J Physiol. 1982 Aug;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Henderson J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987 Mar 5;262(7):3065–3073. [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Morgan J. P. The effects of digitalis on intracellular calcium transients in mammalian working myocardium as detected with aequorin. J Mol Cell Cardiol. 1985 Nov;17(11):1065–1075. doi: 10.1016/s0022-2828(85)80122-x. [DOI] [PubMed] [Google Scholar]
- Moutin M. J., Dupont Y. Rapid filtration studies of Ca2+-induced Ca2+ release from skeletal sarcoplasmic reticulum. Role of monovalent ions. J Biol Chem. 1988 Mar 25;263(9):4228–4235. [PubMed] [Google Scholar]
- Musgrave G. E., Born C. K., Davidson C. P., Hamrick M. E. Interaction of spironolactone and digoxin in dogs. J Pharmacol Exp Ther. 1977 Sep;202(3):696–701. [PubMed] [Google Scholar]
- Noble D. Mechanism of action of therapeutic levels of cardiac glycosides. Cardiovasc Res. 1980 Sep;14(9):495–514. doi: 10.1093/cvr/14.9.495. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990 Apr;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985 Aug 1;316(6027):446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- Tomlins B., Harding S. E., Kirby M. S., Poole-Wilson P. A., Williams A. J. Contamination of a cardiac sarcolemmal preparation with endothelial plasma membrane. Biochim Biophys Acta. 1986 Mar 27;856(1):137–143. doi: 10.1016/0005-2736(86)90020-9. [DOI] [PubMed] [Google Scholar]
- Weingart R., Kass R. S., Tsien R. W. Is digitalis inotropy associated with enhanced slow inward calcium current? Nature. 1978 Jun 1;273(5661):389–392. doi: 10.1038/273389a0. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gen Physiol. 1984 Mar;83(3):395–415. doi: 10.1085/jgp.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J., Holmberg S. R. Sulmazole (AR-L 115BS) activates the sheep cardiac muscle sarcoplasmic reticulum calcium-release channel in the presence and absence of calcium. J Membr Biol. 1990 May;115(2):167–178. doi: 10.1007/BF01869455. [DOI] [PubMed] [Google Scholar]
- Williams A. J. Ion conduction and discrimination in the sarcoplasmic reticulum ryanodine receptor/calcium-release channel. J Muscle Res Cell Motil. 1992 Feb;13(1):7–26. doi: 10.1007/BF01738423. [DOI] [PubMed] [Google Scholar]



