Abstract
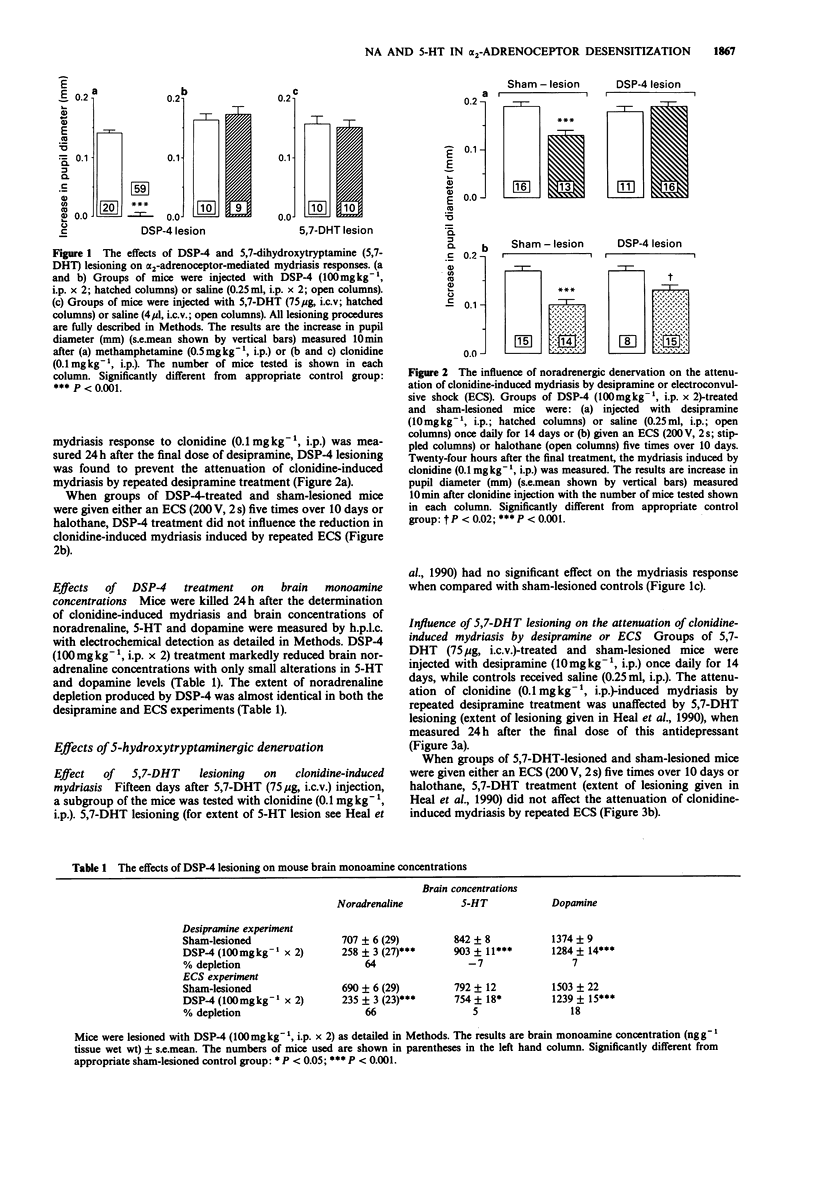
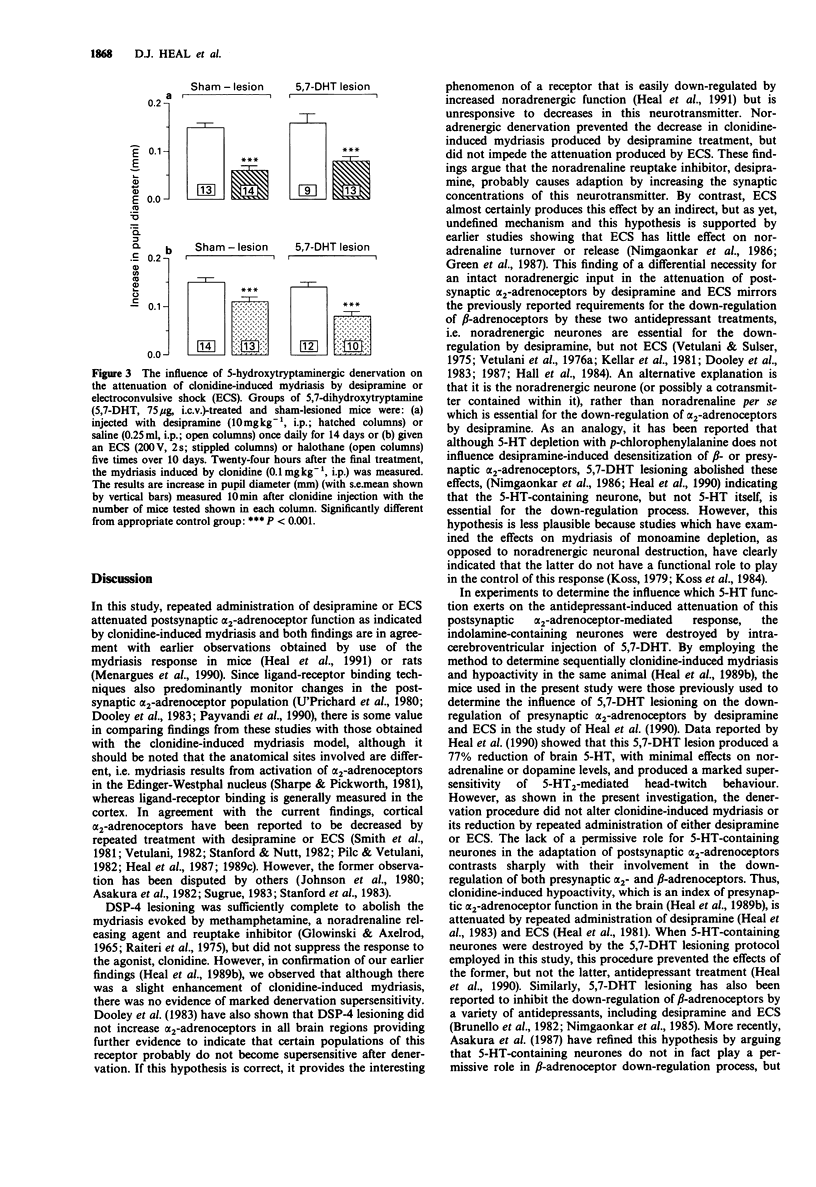
1. Experiments were conducted to determine the respective roles which noradrenergic and 5-hydroxytryptaminergic neurones play in the down-regulation of postsynaptic alpha 2-adrenoceptors by desipramine and electroconvulsive shock (ECS). The functional status of these receptors was monitored by use of clonidine-induced mydriasis in conscious mice. 2. Mydriasis to clonidine (0.1 mg kg-1, i.p.) was markedly attenuated by administration of either desipramine (10 mg kg-1, i.p.) for 14 days or ECS (200 V, 2s) given five times over ten days confirming our previous observations. 3. The neurotoxin, DSP-4 (100 mg kg-1, i.p. X 2), reduced brain noradrenaline levels by 64% and abolished the mydriasis induced by the noradrenaline releasing agent and reuptake inhibitor, methamphetamine, without significantly altering the response to clonidine, confirming our earlier results. This lesion prevented the attenuation of clonidine mydriasis by repeated administration of desipramine, but not ECS. 4. Lesioning of central 5-hydroxytryptaminergic neurones with 5,7-dihydroxytryptamine (75 micrograms, i.c.v.) had no influence on the reduction in clonidine mydriasis produced by repeated administration of either desipramine or ECS. 5. Since noradrenergic neurones are essential for the desensitization of postsynaptic alpha 2-adrenoceptors by desipramine, it indicates that this effect is probably the result of increased synaptic noradrenaline levels. This mechanism is not responsible for the change induced by ECS because this adaptation is independent of an intact noradrenergic input. 5-HT-containing neurones do not play a permissive role in the down-regulation of postsynaptic alpha 2-adrenoceptors by either antidepressant treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura M., Tsukamoto T., Kubota H., Imafuku J., Ino M., Nishizaki J., Sato A., Shinbo K., Hasegawa K. Role of serotonin in the regulation of beta-adrenoceptors by antidepressants. Eur J Pharmacol. 1987 Sep 2;141(1):95–100. doi: 10.1016/0014-2999(87)90414-6. [DOI] [PubMed] [Google Scholar]
- Blackshear M. A., Sanders-Bush E. Serotonin receptor sensitivity after acute and chronic treatment with mianserin. J Pharmacol Exp Ther. 1982 May;221(2):303–308. [PubMed] [Google Scholar]
- Brunello N., Barbaccia M. L., Chuang D. M., Costa E. Down-regulation of beta-adrenergic receptors following repeated injections of desmethylimipramine: permissive role of serotonergic axons. Neuropharmacology. 1982 Nov;21(11):1145–1149. doi: 10.1016/0028-3908(82)90172-1. [DOI] [PubMed] [Google Scholar]
- Brunello N., Volterra A., Cagiano R., Ianieri G. C., Cuomo V., Racagni G. Biochemical and behavioral changes in rats after prolonged treatment with desipramine: interaction with p-chlorophenylalanine. Naunyn Schmiedebergs Arch Pharmacol. 1985 Oct;331(1):20–22. doi: 10.1007/BF00498847. [DOI] [PubMed] [Google Scholar]
- Dooley D. J., Bittiger H., Hauser K. L., Bischoff S. F., Waldmeier P. C. Alteration of central alpha 2- and beta-adrenergic receptors in the rat after DSP-4, a selective noradrenergic neurotoxin. Neuroscience. 1983 Aug;9(4):889–898. doi: 10.1016/0306-4522(83)90277-4. [DOI] [PubMed] [Google Scholar]
- Dooley D. J., Heal D. J., Goodwin G. M. Repeated electroconvulsive shock prevents increased neocortical beta 1-adrenoceptor binding after DSP-4 treatment in rats. Eur J Pharmacol. 1987 Feb 24;134(3):333–337. doi: 10.1016/0014-2999(87)90365-7. [DOI] [PubMed] [Google Scholar]
- GLOWINSKI J., AXELROD J. EFFECT OF DRUGS ON THE UPTAKE, RELEASE, AND METABOLISM OF H3-NOREPINEPHRINE IN THE RAT BRAIN. J Pharmacol Exp Ther. 1965 Jul;149:43–49. [PubMed] [Google Scholar]
- Goodwin G. M., Green A. R., Johnson P. 5-HT2 receptor characteristics in frontal cortex and 5-HT2 receptor-mediated head-twitch behaviour following antidepressant treatment to mice. Br J Pharmacol. 1984 Sep;83(1):235–242. doi: 10.1111/j.1476-5381.1984.tb10140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Heal D. J., Vincent N. D. The effects of single and repeated electroconvulsive shock administration on the release of 5-hydroxytryptamine and noradrenaline from cortical slices of rat brain. Br J Pharmacol. 1987 Sep;92(1):25–30. doi: 10.1111/j.1476-5381.1987.tb11291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H., Ross S. B., Sällemark M. Effect of destruction of central noradrenergic and serotonergic nerve terminals by systemic neurotoxins on the long-term effects of antidepressants on beta-adrenoceptors and 5-HT2 binding sites in the rat cerebral cortex. J Neural Transm. 1984;59(1):9–23. doi: 10.1007/BF01249875. [DOI] [PubMed] [Google Scholar]
- Heal D. J., Akagi H., Bowdler J. M., Green A. R. Repeated electroconvulsive shock attenuates clonidine-induced hypoactivity in rodents. Eur J Pharmacol. 1981 Nov 5;75(4):231–237. doi: 10.1016/0014-2999(81)90549-5. [DOI] [PubMed] [Google Scholar]
- Heal D. J., Bristow L. J., Elliott J. M., Bloomfield J. G., Catto L. C., Atterwill C. K. The influence of L-triiodothyronine (T3) on the effects of repeated administration of desipramine or electroconvulsive shock on alpha 2- and beta-adrenoceptor function in the brain of the rat: implications for the potentiation of antidepressant therapy by T3. Neuropharmacology. 1987 Aug;26(8):1131–1139. doi: 10.1016/0028-3908(87)90259-0. [DOI] [PubMed] [Google Scholar]
- Heal D. J., Bristow L. M., Hurst E. M., Elliott J. M., Buckett W. R. Sex-related differences in central adrenergic function and responsiveness to repeated administration of desipramine or electroconvulsive shock. Br J Pharmacol. 1989 May;97(1):111–118. doi: 10.1111/j.1476-5381.1989.tb11930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal D. J., Hurst E. M., Prow M. R., Buckett W. R. An investigation of the role of 5-hydroxytryptamine in the attenuation of presynaptic alpha 2-adrenoceptor-mediated responses by antidepressant treatments. Psychopharmacology (Berl) 1990;101(1):100–106. doi: 10.1007/BF02253725. [DOI] [PubMed] [Google Scholar]
- Heal D. J., Lister S., Smith S. L., Davies C. L., Molyneux S. G., Green A. R. The effects of acute and repeated administration of various antidepressant drugs on clonidine-induced hypoactivity in mice and rats. Neuropharmacology. 1983 Aug;22(8):983–992. doi: 10.1016/0028-3908(83)90214-9. [DOI] [PubMed] [Google Scholar]
- Heal D. J. Phenylephrine-induced activity in mice as a model of central alpha 1-adrenoceptor function. Effects of acute and repeated administration of antidepressant drugs and electroconvulsive shock. Neuropharmacology. 1984 Nov;23(11):1241–1251. doi: 10.1016/0028-3908(84)90040-6. [DOI] [PubMed] [Google Scholar]
- Heal D. J., Prow M. R., Buckett W. R. Clonidine produces mydriasis in conscious mice by activating central alpha 2-adrenoceptors. Eur J Pharmacol. 1989 Oct 24;170(1-2):11–18. doi: 10.1016/0014-2999(89)90127-1. [DOI] [PubMed] [Google Scholar]
- Heal D. J., Prow M. R., Buckett W. R. Effects of antidepressant drugs and electroconvulsive shock on pre- and postsynaptic alpha 2-adrenoceptor function in the brain: rapid down-regulation by sibutramine hydrochloride. Psychopharmacology (Berl) 1991;103(2):251–257. doi: 10.1007/BF02244212. [DOI] [PubMed] [Google Scholar]
- Johnson R. W., Reisine T., Spotnitz S., Wiech N., Ursillo R., Yamamura H. I. Effects of desipramine and yohimbine on alpha 2- and beta-adrenoreceptor sensitivity. Eur J Pharmacol. 1980 Oct 3;67(1):123–127. doi: 10.1016/0014-2999(80)90019-9. [DOI] [PubMed] [Google Scholar]
- Kellar K. J., Cascio C. S., Bergstrom D. A., Butler J. A., Iadarola P. Electroconvulsive shock and reserpine: effects on beta-adrenergic receptors in rat brain. J Neurochem. 1981 Oct;37(4):830–836. doi: 10.1111/j.1471-4159.1981.tb04468.x. [DOI] [PubMed] [Google Scholar]
- Koss M. C. Clonidine mydriasis in the cat. Further evidence for a CNS postsynaptic action. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(3):235–239. doi: 10.1007/BF00504755. [DOI] [PubMed] [Google Scholar]
- Koss M. C., Gherezghiher T., Nomura A. CNS adrenergic inhibition of parasympathetic oculomotor tone. J Auton Nerv Syst. 1984 Mar;10(1):55–68. doi: 10.1016/0165-1838(84)90067-5. [DOI] [PubMed] [Google Scholar]
- Menargues A., Obach R., García-Sevilla J. A. Modulation by antidepressant drugs of CNS postsynaptic alpha 2-adrenoceptors mediating mydriasis in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):101–107. doi: 10.1007/BF00195065. [DOI] [PubMed] [Google Scholar]
- Metz A., Heal D. J. In mice repeated administration of electroconvulsive shock or desmethylimipramine produces rapid alterations in 5-HT2-mediated head-twitch responses and cortical 5-HT2 receptor number. Eur J Pharmacol. 1986 Jul 15;126(1-2):159–162. doi: 10.1016/0014-2999(86)90754-5. [DOI] [PubMed] [Google Scholar]
- Nimgaonkar V. L., Goodwin G. M., Davies C. L., Green A. R. Down-regulation of beta-adrenoceptors in rat cortex by repeated administration of desipramine, electroconvulsive shock and clenbuterol requires 5-HT neurones but not 5-HT. Neuropharmacology. 1985 Apr;24(4):279–283. doi: 10.1016/0028-3908(85)90132-7. [DOI] [PubMed] [Google Scholar]
- Nimgaonkar V. L., Heal D. J., Davies C. L., Green A. R. Studies on rat brain catecholamine synthesis and beta-adrenoceptor number following administration of electroconvulsive shock, desipramine and clenbuterol. J Neural Transm. 1986;65(3-4):245–259. doi: 10.1007/BF01249086. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Snyder S. H. Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science. 1980 Oct 3;210(4465):88–90. doi: 10.1126/science.6251550. [DOI] [PubMed] [Google Scholar]
- Pilc A., Vetulani J. Depression by chronic electroconvulsive treatment of clonidine hypotherma and [3H]clonidine binding to rat cortical membranes. Eur J Pharmacol. 1982 May 7;80(1):109–113. doi: 10.1016/0014-2999(82)90184-4. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Bertollini A., Angelini F., Levi G. d-Amphetamine as a releaser or reuptake inhibitor of biogenic amines in synaptosomes. Eur J Pharmacol. 1975 Nov;34(1):189–195. doi: 10.1016/0014-2999(75)90239-3. [DOI] [PubMed] [Google Scholar]
- Schultz J. Psychoactive drug effects on a system which generates cyclic AMP in brain. Nature. 1976 Jun 3;261(5559):417–418. doi: 10.1038/261417a0. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Garcia-Sevilla J. A., Hollingsworth P. J. alpha 2-Adrenoreceptors in rat brain are decreased after long-term tricyclic antidepressant drug treatment. Brain Res. 1981 Apr 6;210(1-2):413–418. doi: 10.1016/0006-8993(81)90919-7. [DOI] [PubMed] [Google Scholar]
- Stanford C., Nutt D. J., Cowen P. J. Comparison of the effects of chronic desmethylimipramine administration on alpha 2- and beta-adrenoceptors in different regions of rat brain. Neuroscience. 1983 Jan;8(1):161–164. doi: 10.1016/0306-4522(83)90035-0. [DOI] [PubMed] [Google Scholar]
- Stanford S. C., Nutt D. J. Comparison of the effects of repeated electroconvulsive shock on alpha 2- and beta-adrenoceptors in different regions of rat brain. Neuroscience. 1982 Jul;7(7):1753–1757. doi: 10.1016/0306-4522(82)90032-x. [DOI] [PubMed] [Google Scholar]
- Sugrue M. F. Some effects of chronic antidepressant treatments on rat brain monoaminergic systems. J Neural Transm. 1983;57(4):281–295. doi: 10.1007/BF01248999. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Reisine T. D., Mason S. T., Fibiger H. C., Yamamura H. I. Modulation of rat brain alpha- and beta-adrenergic receptor populations by lesion of the dorsal noradrenergic bundle. Brain Res. 1980 Apr 7;187(1):143–154. doi: 10.1016/0006-8993(80)90500-4. [DOI] [PubMed] [Google Scholar]
- Vetulani J., Stawarz R. J., Dingell J. V., Sulser F. A possible common mechanism of action of antidepressant treatments: reduction in the sensitivity of the noradrenergic cyclic AMP gererating system in the rat limbic forebrain. Naunyn Schmiedebergs Arch Pharmacol. 1976 May;293(2):109–114. doi: 10.1007/BF00499215. [DOI] [PubMed] [Google Scholar]
- Vetulani J., Stawarz R. J., Sulser F. Adaptive mechanisms of the noradrenergic cyclic AMP generating system in the limbic forebrain of the rat: adaptation to persistent changes in the availability of norepinephrine (NE). J Neurochem. 1976 Sep;27(3):661–666. doi: 10.1111/j.1471-4159.1976.tb10391.x. [DOI] [PubMed] [Google Scholar]
- Vetulani J., Sulser F. Action of various antidepressant treatments reduces reactivity of noradrenergic cyclic AMP-generating system in limbic forebrain. Nature. 1975 Oct 9;257(5526):495–496. doi: 10.1038/257495a0. [DOI] [PubMed] [Google Scholar]


