Abstract
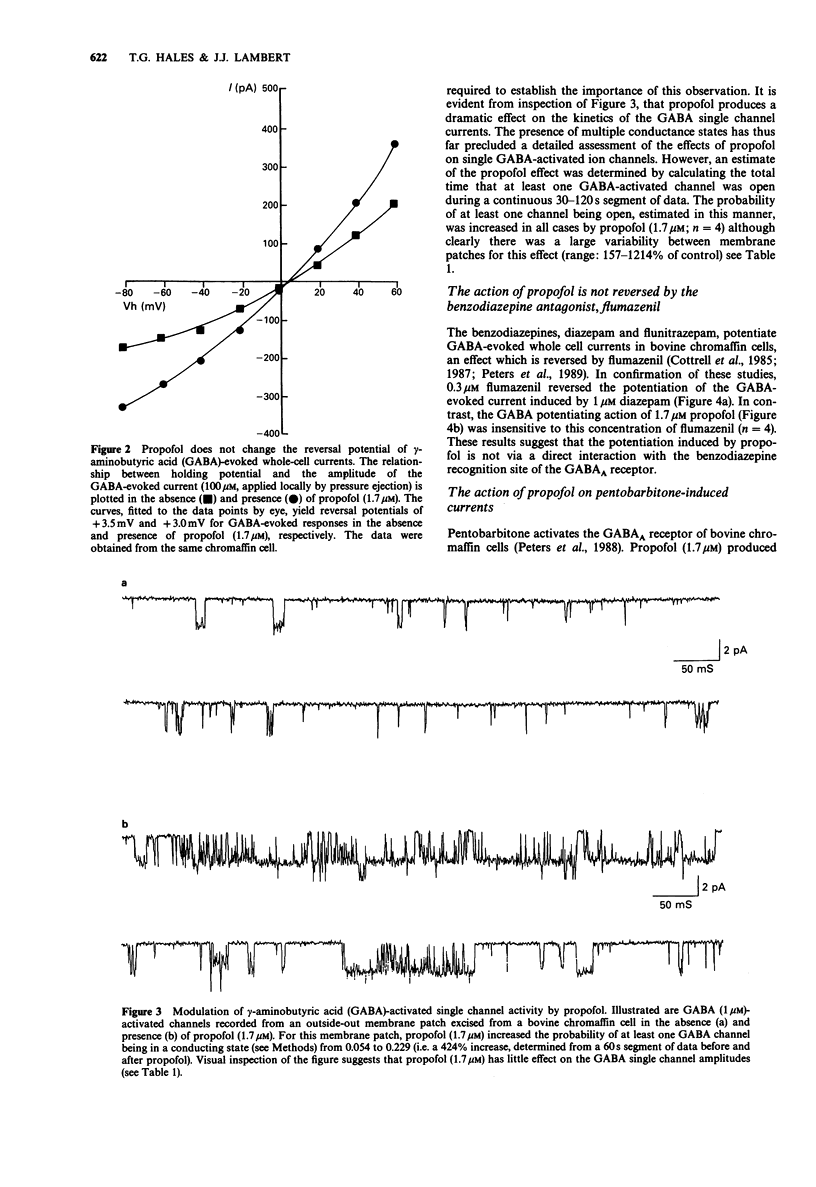
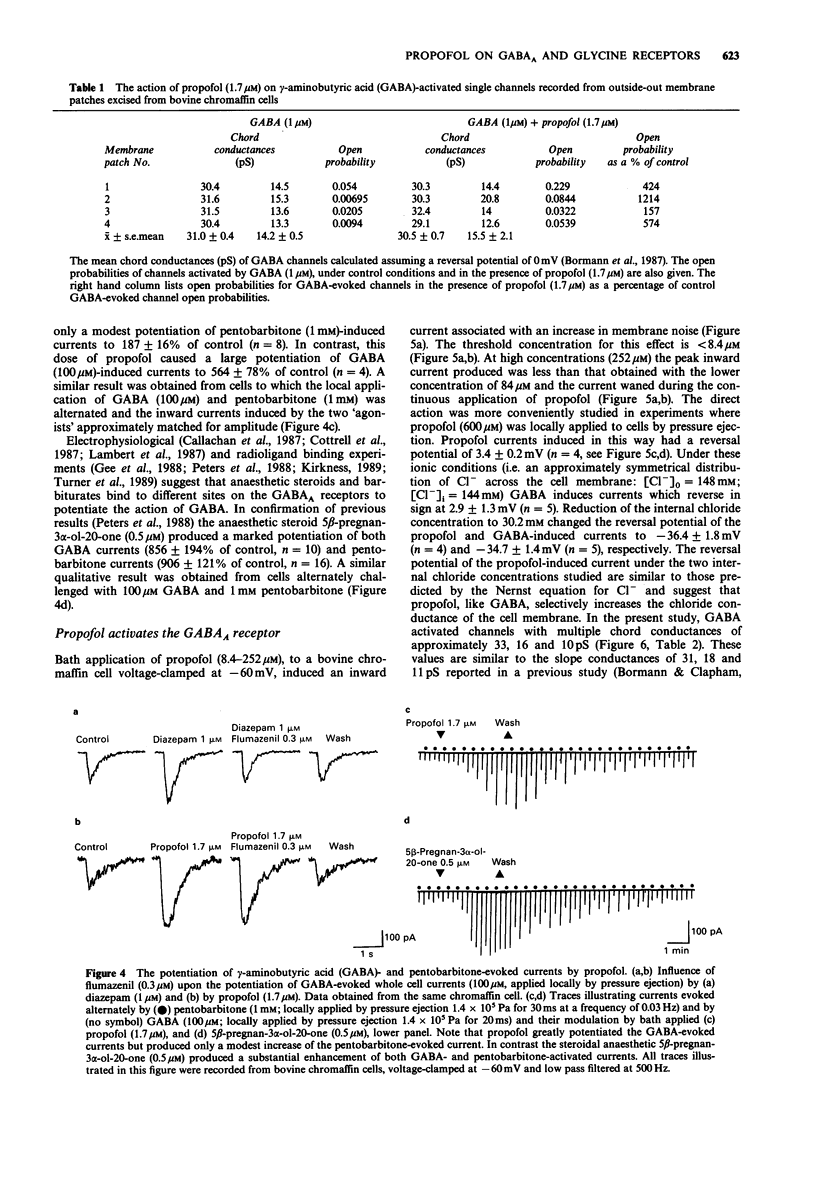
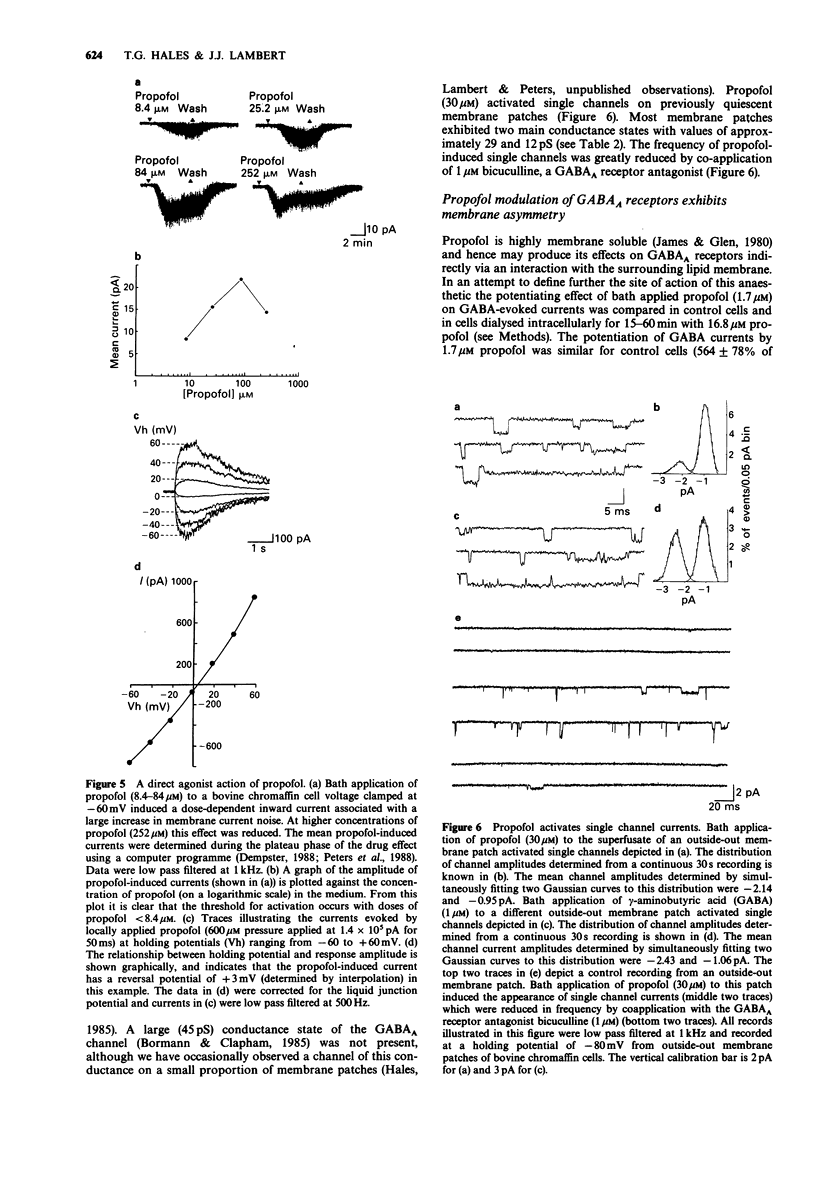
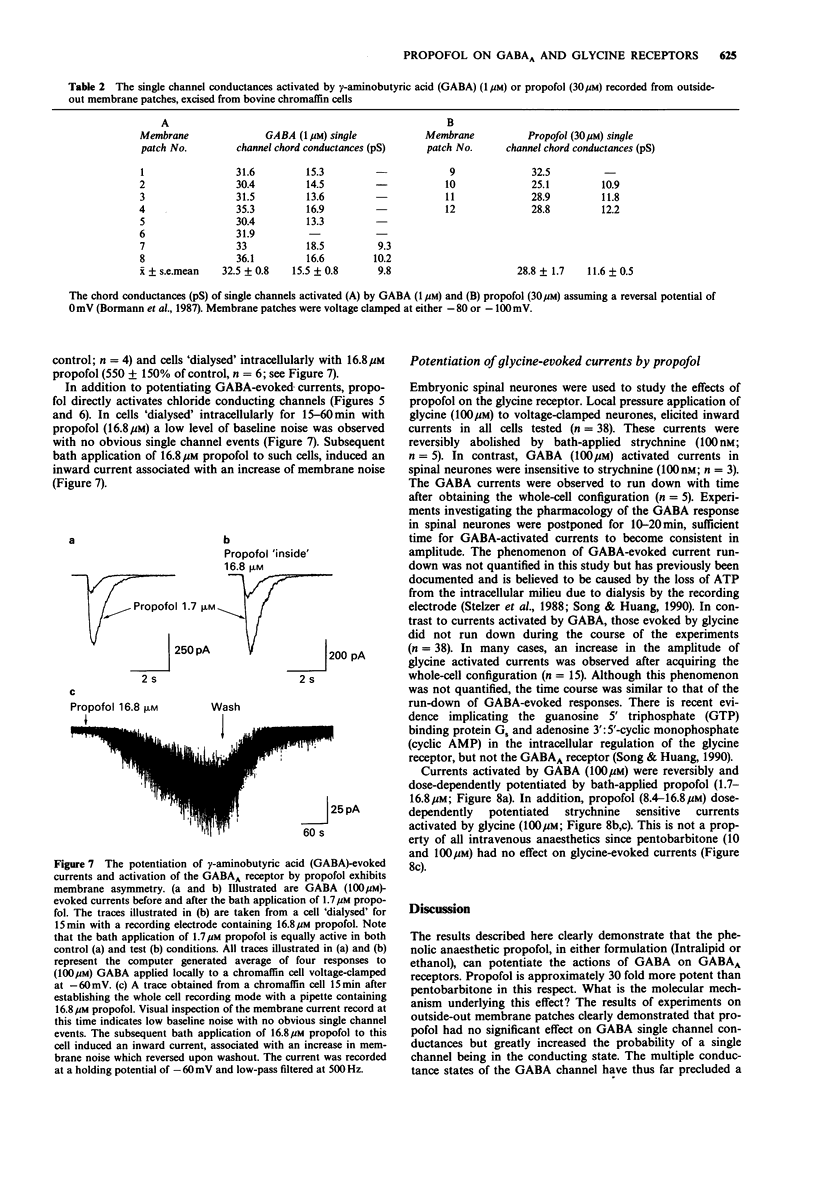
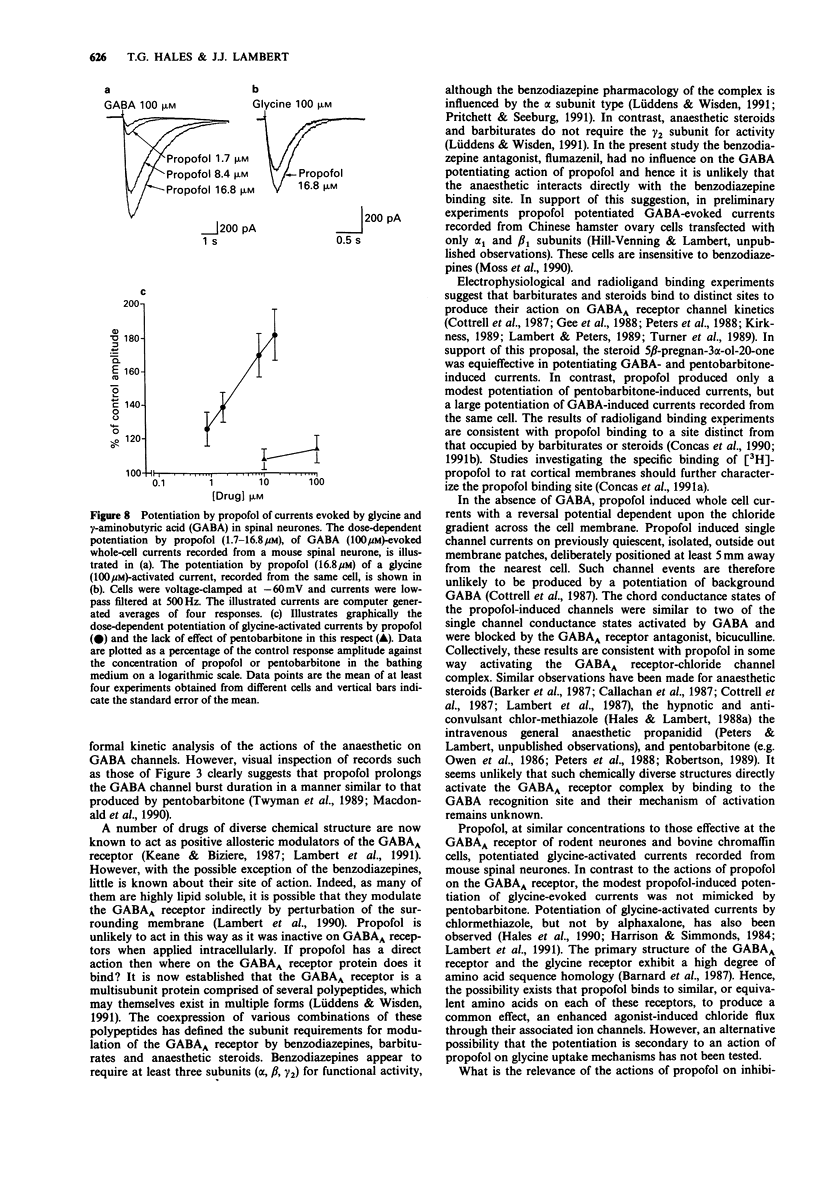
1. The interaction of the intravenous general anaesthetic propofol (2,6-diisopropylphenol) with the GABAA receptor has been investigated in voltage-clamped bovine chromaffin cells and rat cortical neurones in cell culture. Additionally, the effects of propofol on the glycine and GABAA receptors of murine spinal neurones were determined. 2. Propofol (1.7-16.8 microM) reversibly and dose-dependently potentiated the amplitude of membrane currents elicited by GABA (100 microM) applied locally to bovine chromaffin cells. Intracellular application of propofol (16.8 microM) was ineffective. In rat cortical neurones and murine spinal neurones, extracellular application of 8.4 microM and 1.7-16.8 microM propofol respectively produced a potentiation of GABA-evoked currents qualitatively similar to that seen in the bovine chromaffin cell. 3. The potentiation by propofol (1.7 microM) was not associated with a change in the reversal potential of the GABA-evoked whole cell current. On outside-out membrane patches isolated from bovine chromaffin cells, propofol (1.7 microM) had little or no effect on the GABA single channel conductances, but greatly increased the probability of the GABA-gated channel being in the conducting state. 4. The potentiation of GABA-evoked whole cell currents by propofol (1.7 microM) was not influenced by the benzodiazepine antagonist flumazenil (0.3 microM). A concentration of propofol (1.7 microM) that substantially potentiated GABA currents had little effect on currents induced by the activation of the GABAA receptor by pentobarbitone (1 mM). 5. Bath application of propofol (8.4-252 microM), to bovine chromaffin cells voltage clamped at -60 mV, induced an inward current associated with an increase in membrane current noise on all cells sensitive to GABA. Intracellular application of propofol (16.8 microM) was ineffective in this respect. Local application of propofol (600 microM) induced whole cell currents with a reversal potential dependent upon the Cl- gradient across the cell membrane. 6. On outside-out membrane patches formed from bovine chromaffin cells, propofol (30 microM) induced single channels with mean chord conductances of 29 and 12 pS. The frequency of propofol channels was greatly reduced by coapplication of 1 microM bicuculline. Under identical ionic conditions, GABA (1 microM) activated single channels with mean chord conductances of 33, 16 and 10pS. 7. Bath applied propofol (0.84-16.8 microM) dose-dependently potentiated strychnine-sensitive currents evoked by glycine (100 microM) in murine spinal neurones. 8. The relevance of the present results to the general anaesthetic action of propofol is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Harrison N. L., Lange G. D., Owen D. G. Potentiation of gamma-aminobutyric-acid-activated chloride conductance by a steroid anaesthetic in cultured rat spinal neurones. J Physiol. 1987 May;386:485–501. doi: 10.1113/jphysiol.1987.sp016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., MacDonald J. F. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982 Jan;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callachan H., Cottrell G. A., Hather N. Y., Lambert J. J., Nooney J. M., Peters J. A. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987 Aug 21;231(1264):359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Coates D. P., Prys-Roberts C., Spelina K. R., Monk C. R., Norley I. Propofol ('Diprivan') by intravenous infusion with nitrous oxide: dose requirements and haemodynamic effects. Postgrad Med J. 1985;61 (Suppl 3):76–79. [PubMed] [Google Scholar]
- Collins G. G. Effects of the anaesthetic 2,6-diisopropylphenol on synaptic transmission in the rat olfactory cortex slice. Br J Pharmacol. 1988 Nov;95(3):939–949. doi: 10.1111/j.1476-5381.1988.tb11724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A., Santoro G., Mascia M. P., Serra M., Sanna E., Biggio G. The general anesthetic propofol enhances the function of gamma-aminobutyric acid-coupled chloride channel in the rat cerebral cortex. J Neurochem. 1990 Dec;55(6):2135–2138. doi: 10.1111/j.1471-4159.1990.tb05807.x. [DOI] [PubMed] [Google Scholar]
- Concas A., Santoro G., Serra M., Sanna E., Biggio G. Neurochemical action of the general anaesthetic propofol on the chloride ion channel coupled with GABAA receptors. Brain Res. 1991 Mar 1;542(2):225–232. doi: 10.1016/0006-8993(91)91571-h. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A., Lambert J. J., Peters J. A. Modulation of GABAA receptor activity by alphaxalone. Br J Pharmacol. 1987 Mar;90(3):491–500. doi: 10.1111/j.1476-5381.1987.tb11198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985 Jul;85(3):675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee K. W., Bolger M. B., Brinton R. E., Coirini H., McEwen B. S. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988 Aug;246(2):803–812. [PubMed] [Google Scholar]
- Gent J. P., Wacey T. A. The effects of chlormethiazole on single unit activity in rat brain; interactions with inhibitory and excitatory neurotransmitters. Br J Pharmacol. 1983 Nov;80(3):439–444. doi: 10.1111/j.1476-5381.1983.tb10713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glen J. B. Animal studies of the anaesthetic activity of ICI 35 868. Br J Anaesth. 1980 Aug;52(8):731–742. doi: 10.1093/bja/52.8.731. [DOI] [PubMed] [Google Scholar]
- Halliwell R. F., Peters J. A., Lambert J. J. The mechanism of action and pharmacological specificity of the anticonvulsant NMDA antagonist MK-801: a voltage clamp study on neuronal cells in culture. Br J Pharmacol. 1989 Feb;96(2):480–494. doi: 10.1111/j.1476-5381.1989.tb11841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N. L., Majewska M. D., Harrington J. W., Barker J. L. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987 Apr;241(1):346–353. [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984 Dec 10;323(2):287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Glen J. B. Synthesis, biological evaluation, and preliminary structure-activity considerations of a series of alkylphenols as intravenous anesthetic agents. J Med Chem. 1980 Dec;23(12):1350–1357. doi: 10.1021/jm00186a013. [DOI] [PubMed] [Google Scholar]
- Kataoka Y., Gutman Y., Guidotti A., Panula P., Wroblewski J., Cosenza-Murphy D., Wu J. Y., Costa E. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane P. E., Biziere K. The effects of general anaesthetics on GABAergic synaptic transmission. Life Sci. 1987 Sep 21;41(12):1437–1448. doi: 10.1016/0024-3205(87)90708-9. [DOI] [PubMed] [Google Scholar]
- Kirkness E. F. Steroid modulation reveals further complexity of GABAA receptors. Trends Pharmacol Sci. 1989 Jan;10(1):6–7. doi: 10.1016/0165-6147(89)90091-6. [DOI] [PubMed] [Google Scholar]
- Kirkness E. F., Turner A. J. The gamma-aminobutyrate/benzodiazepine receptor from pig brain. Enhancement of gamma-aminobutyrate-receptor binding by the anaesthetic propanidid. Biochem J. 1986 Jan 1;233(1):259–264. doi: 10.1042/bj2330259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. J., Peters J. A., Sturgess N. C., Hales T. G. Steroid modulation of the GABAA receptor complex: electrophysiological studies. Ciba Found Symp. 1990;153:56–82. doi: 10.1002/9780470513989.ch4. [DOI] [PubMed] [Google Scholar]
- Langley M. S., Heel R. C. Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic. Drugs. 1988 Apr;35(4):334–372. doi: 10.2165/00003495-198835040-00002. [DOI] [PubMed] [Google Scholar]
- Lodge D., Anis N. A. Effects of ketamine and three other anaesthetics on spinal reflexes and inhibitions in the cat. Br J Anaesth. 1984 Oct;56(10):1143–1151. doi: 10.1093/bja/56.10.1143. [DOI] [PubMed] [Google Scholar]
- Lüddens H., Wisden W. Function and pharmacology of multiple GABAA receptor subunits. Trends Pharmacol Sci. 1991 Feb;12(2):49–51. doi: 10.1016/0165-6147(91)90495-e. [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Rogers C. J., Twyman R. E. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. 1989 Oct;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Barker J. L. Anticonvulsant and anesthetic barbiturates: different postsynaptic actions in cultured mammalian neurons. Neurology. 1979 Apr;29(4):432–447. doi: 10.1212/wnl.29.4.432. [DOI] [PubMed] [Google Scholar]
- Mackenzie N., Grant I. S. Propofol ('Diprivan') for continuous intravenous anaesthesia. A comparison with methohexitone. Postgrad Med J. 1985;61 (Suppl 3):70–75. [PubMed] [Google Scholar]
- Moss S. J., Smart T. G., Porter N. M., Nayeem N., Devine J., Stephenson F. A., Macdonald R. L., Barnard E. A. Cloned GABA receptors are maintained in a stable cell line: allosteric and channel properties. Eur J Pharmacol. 1990 Jul 31;189(1):77–88. doi: 10.1016/0922-4106(90)90232-m. [DOI] [PubMed] [Google Scholar]
- Nakahiro M., Yeh J. Z., Brunner E., Narahashi T. General anesthetics modulate GABA receptor channel complex in rat dorsal root ganglion neurons. FASEB J. 1989 May;3(7):1850–1854. doi: 10.1096/fasebj.3.7.2541038. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Kirkness E. F., Callachan H., Lambert J. J., Turner A. J. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br J Pharmacol. 1988 Aug;94(4):1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. A., Lambert J. J., Cottrell G. A. An electrophysiological investigation of the characteristics and function of GABAA receptors on bovine adrenomedullary chromaffin cells. Pflugers Arch. 1989 Oct;415(1):95–103. doi: 10.1007/BF00373146. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Seeburg P. H. gamma-Aminobutyric acid type A receptor point mutation increases the affinity of compounds for the benzodiazepine site. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1421–1425. doi: 10.1073/pnas.88.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Smaje J. C. Anaesthetics depress the sensitivity of cortical neurones to L-glutamate. Br J Pharmacol. 1976 Nov;58(3):347–357. [PMC free article] [PubMed] [Google Scholar]
- Robertson B. Actions of anaesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurones. Br J Pharmacol. 1989 Sep;98(1):167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds M. A., Turner J. P. Potentiators of responses to activation of gamma-aminobutyric acid (GABAA) receptors. Neuropharmacology. 1987 Jul;26(7B):923–930. doi: 10.1016/0028-3908(87)90071-2. [DOI] [PubMed] [Google Scholar]
- Song Y. M., Huang L. Y. Modulation of glycine receptor chloride channels by cAMP-dependent protein kinase in spinal trigeminal neurons. Nature. 1990 Nov 15;348(6298):242–245. doi: 10.1038/348242a0. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Tal N., Goldberg O., Luini A. Barbiturates, alcohols and the CNS excitatory neurotransmission: specific effects on the kainate and quisqualate receptors. Brain Res. 1984 Jan 23;291(2):285–292. doi: 10.1016/0006-8993(84)91260-5. [DOI] [PubMed] [Google Scholar]
- Turner D. M., Ransom R. W., Yang J. S., Olsen R. W. Steroid anesthetics and naturally occurring analogs modulate the gamma-aminobutyric acid receptor complex at a site distinct from barbiturates. J Pharmacol Exp Ther. 1989 Mar;248(3):960–966. [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Pentobarbital and picrotoxin have reciprocal actions on single GABAA receptor channels. Neurosci Lett. 1989 Jan 2;96(1):89–95. doi: 10.1016/0304-3940(89)90248-6. [DOI] [PubMed] [Google Scholar]
- de Grood P. M., Ruys A. H., van Egmond J., Booij L. H., Crul J. F. Propofol ('Diprivan') emulsion for total intravenous anaesthesia. Postgrad Med J. 1985;61 (Suppl 3):65–69. [PubMed] [Google Scholar]



