Abstract
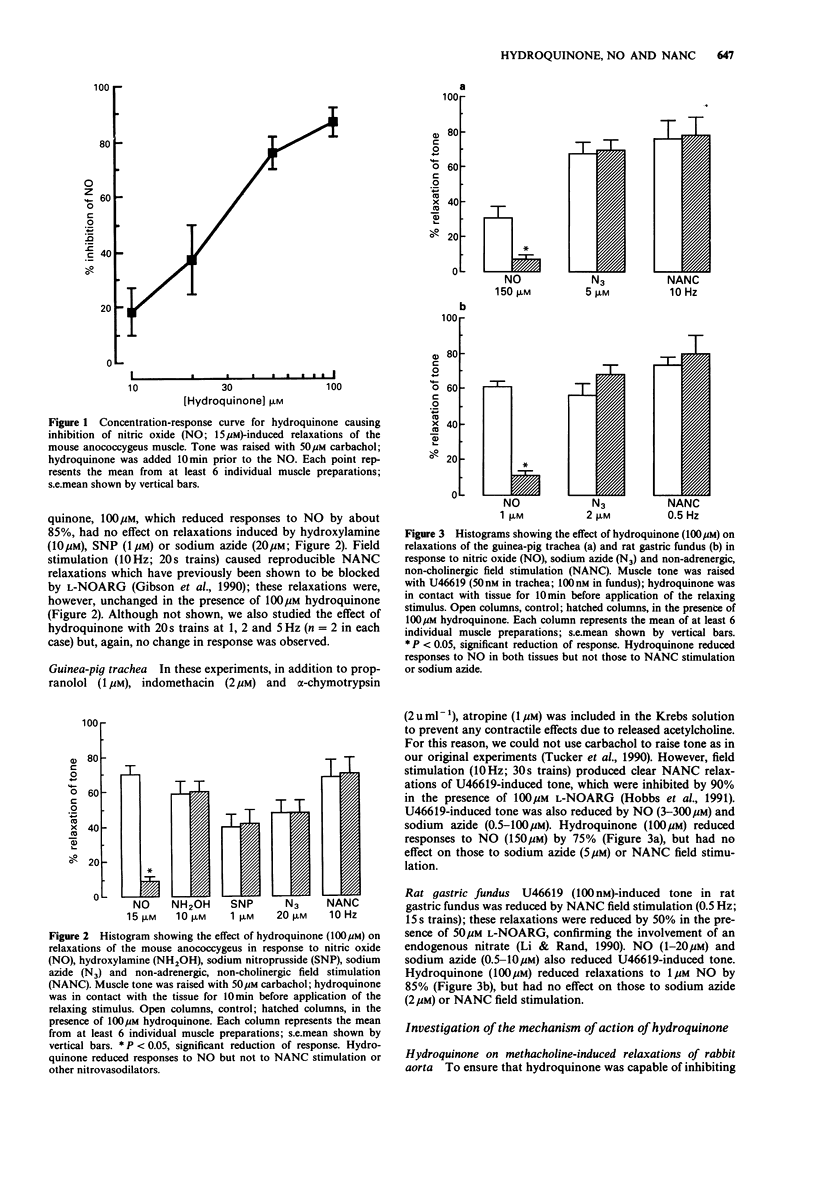
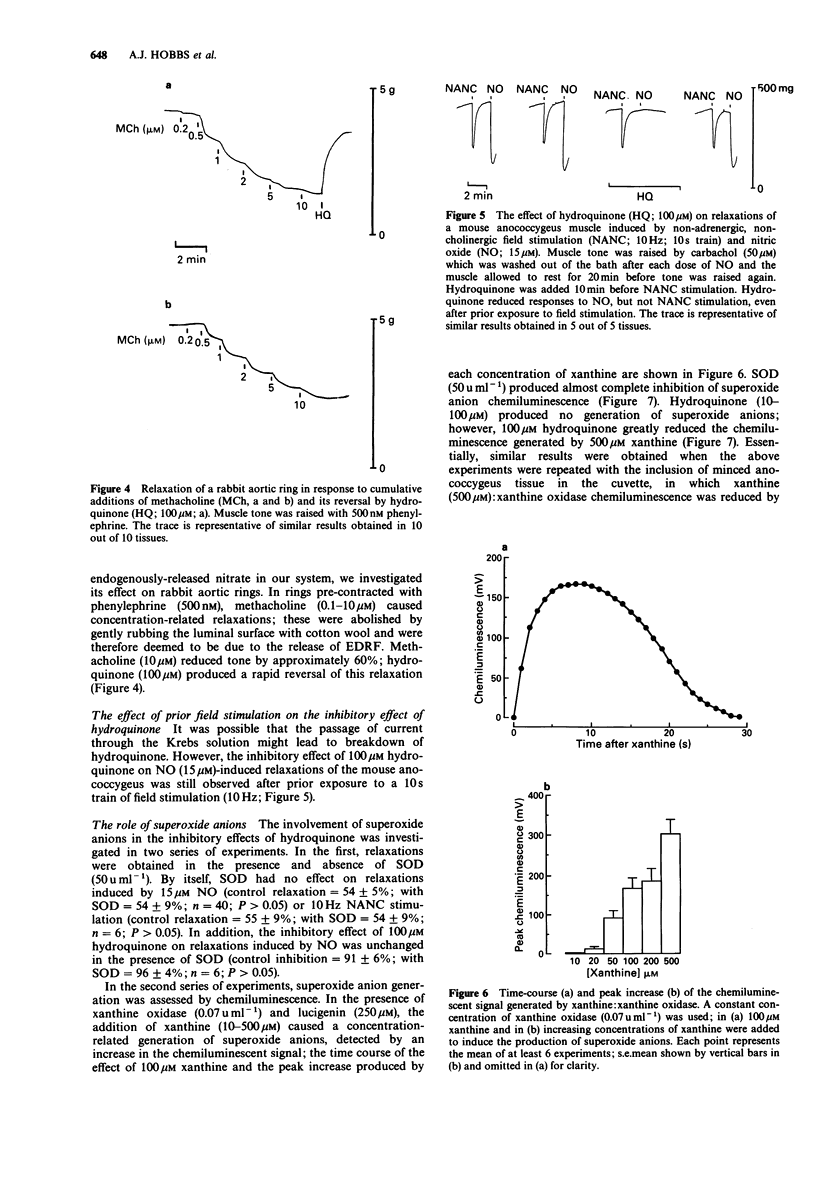
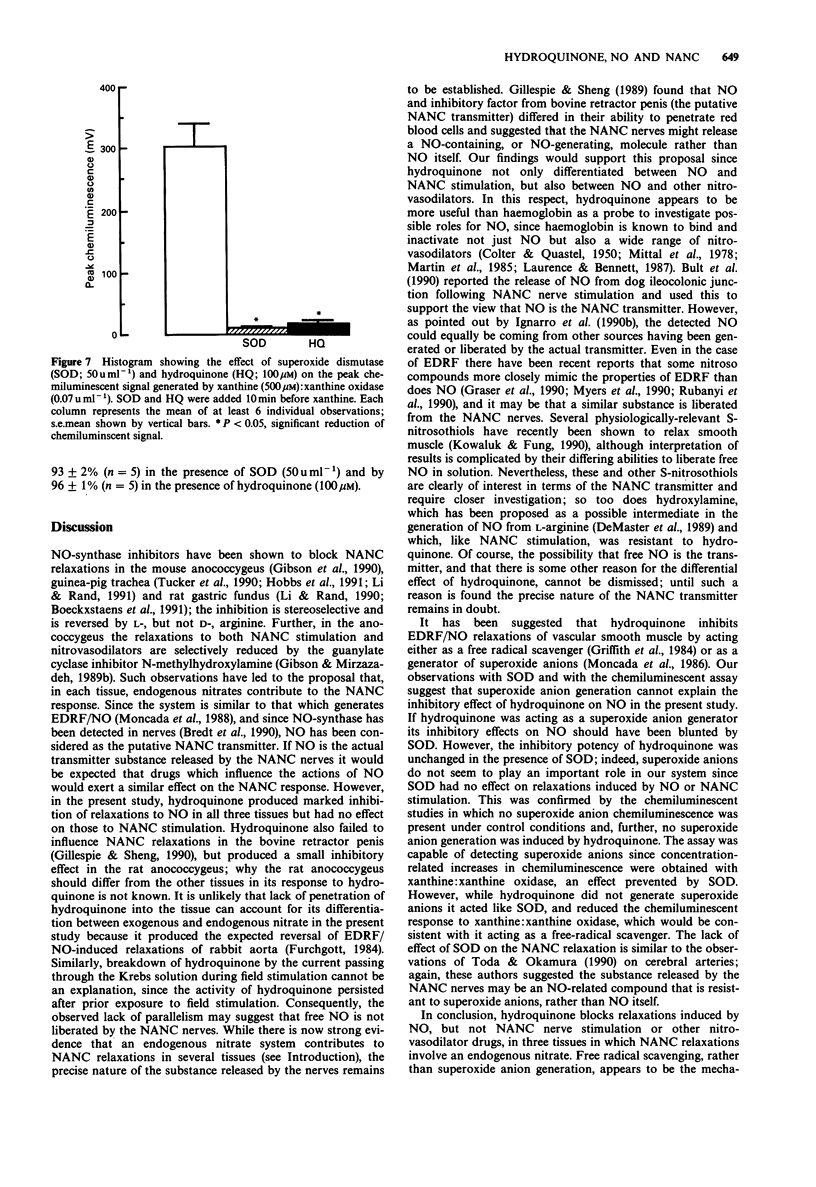
1. The influence of hydroquinone on relaxations induced by nitric oxide (NO), nitrovasodilator drugs, and non-adrenergic, non-cholinergic (NANC) field stimulation has been investigated in three tissues in which endogenous nitrates have been implicated in the NANC response; the mechanism of action of hydroquinone was also studied. 2. In mouse anococcygeus, hydroquinone (10-100 microM) produced a concentration-dependent inhibition of relaxations induced by 15 microM NO. Hydroquinone, 100 microM, which reduced responses to NO by 85%, had no effect on relaxations induced by NANC field stimulation (10 Hz; 20s trains), hydroxylamine (10 microM), sodium nitroprusside (1 microM) or sodium azide (20 microM). 3. In guinea-pig trachea, 100 microM hydroquinone reduced relaxations to 150 microM NO by 75%, but had no effect on those to NANC stimulation (10 Hz; 30 s trains) or sodium azide (5 microM). 4. In rat gastric fundus, 100 microM hydroquinone reduced relaxations to 1 microM NO by 85%, but had no effect on those to NANC stimulation (0.5 Hz; 15 s trains) or sodium azide (2 microM). 5. Superoxide dismutase (SOD; 50 u ml-1) had no effect on relaxations of the mouse anococcygeus in response to 15 microM NO or 10 Hz NANC stimulation. Further, the inhibition of responses to NO by hydroquinone was unaffected in the presence of SOD. 6. Hydroquinone (10-100 microM) failed to generate superoxide anions, as detected by a chemiluminescent assay. However, 100 microM hydroquinone, like SOD (50 u ml-1), produced almost complete inhibition of superoxide anion chemiluminescence induced by xanthine (500 microM): xanthine oxidase (0.07 u ml-1). 7. It is concluded that, in our system, hydroquinone inhibits NO by acting as a free radical scavenger rather than by generating superoxide anions. The ability of hydroquinone to block relaxations to NO, but not NANC stimulation, may suggest that the endogenous nitrate substance released by these NANC nerves may not be free NO, but may be an NO-containing, or NO-generating, molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K. E., Garcia Pascual A., Forman A., Tøttrup A. Non-adrenergic, non-cholinergic nerve-mediated relaxation of rabbit urethra is caused by nitric oxide. Acta Physiol Scand. 1991 Jan;141(1):133–134. doi: 10.1111/j.1748-1716.1991.tb09056.x. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Bogers J. J., Bult H., De Man J. G., Oosterbosch L., Herman A. G., Van Maercke Y. M. Release of nitric oxide upon stimulation of nonadrenergic noncholinergic nerves in the rat gastric fundus. J Pharmacol Exp Ther. 1991 Feb;256(2):441–447. [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Bult H., De Man J. G., Herman A. G., Van Maercke Y. M. Non-adrenergic non-cholinergic relaxation mediated by nitric oxide in the canine ileocolonic junction. Eur J Pharmacol. 1990 Nov 6;190(1-2):239–246. doi: 10.1016/0014-2999(90)94132-h. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- COLTER J. S., QUASTEL J. H. Catalytic decomposition of hydroxylamine by hemoglobin. Arch Biochem. 1950 Jul;27(2):368–389. [PubMed] [Google Scholar]
- DeMaster E. G., Raij L., Archer S. L., Weir E. K. Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of L-arginine to nitric oxide. Biochem Biophys Res Commun. 1989 Aug 30;163(1):527–533. doi: 10.1016/0006-291x(89)92169-4. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs. Br J Pharmacol. 1989 Mar;96(3):637–644. doi: 10.1111/j.1476-5381.1989.tb11863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. Nonpurine, nonpeptide mechanisms. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:20–29. [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. A comparison of haemoglobin and erythrocytes as inhibitors of smooth muscle relaxation by the NANC transmitter in the BRP and rat anococcygeus and by EDRF in the rabbit aortic strip. Br J Pharmacol. 1989 Oct;98(2):445–450. doi: 10.1111/j.1476-5381.1989.tb12616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscles. Br J Pharmacol. 1990 Jan;99(1):194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist F., Hedlund H., Andersson K. E. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxation of human isolated corpus cavernosum. Acta Physiol Scand. 1991 Mar;141(3):441–442. doi: 10.1111/j.1748-1716.1991.tb09103.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Bush P. A., Buga G. M., Rajfer J. Neurotransmitter identity doubt. Nature. 1990 Sep 13;347(6289):131–132. doi: 10.1038/347131b0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Bush P. A., Buga G. M., Wood K. S., Fukuto J. M., Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990 Jul 31;170(2):843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br J Pharmacol. 1991 Jan;102(1):91–94. doi: 10.1111/j.1476-5381.1991.tb12137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur J Pharmacol. 1990 Dec 4;191(3):303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Mittal C. K., Arnold W. P., Murad F. Characterization of protein inhibitors of guanylate cyclase activation from rat heart and bovine lung. J Biol Chem. 1978 Feb 25;253(4):1266–1271. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Gryglewski R. J. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Ramagopal M. V., Leighton H. J. Effects of NG-monomethyl-L-arginine on field stimulation-induced decreases in cytosolic Ca2+ levels and relaxation in the rat anococcygeus muscle. Eur J Pharmacol. 1989 Dec 19;174(2-3):297–299. doi: 10.1016/0014-2999(89)90325-7. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Possible role of nitric oxide in transmitting information from vasodilator nerve to cerebroarterial muscle. Biochem Biophys Res Commun. 1990 Jul 16;170(1):308–313. doi: 10.1016/0006-291x(90)91275-w. [DOI] [PubMed] [Google Scholar]
- Tucker J. F., Brave S. R., Charalambous L., Hobbs A. J., Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990 Aug;100(4):663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


