Abstract
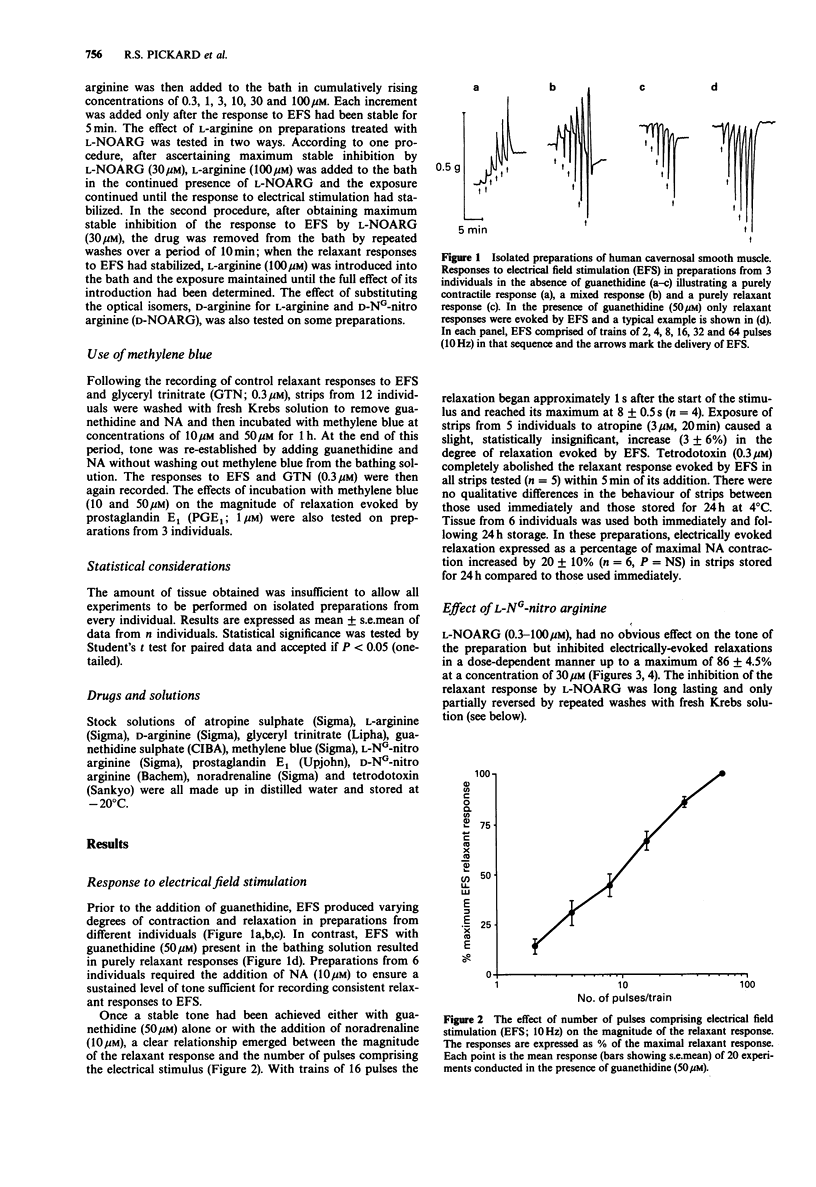
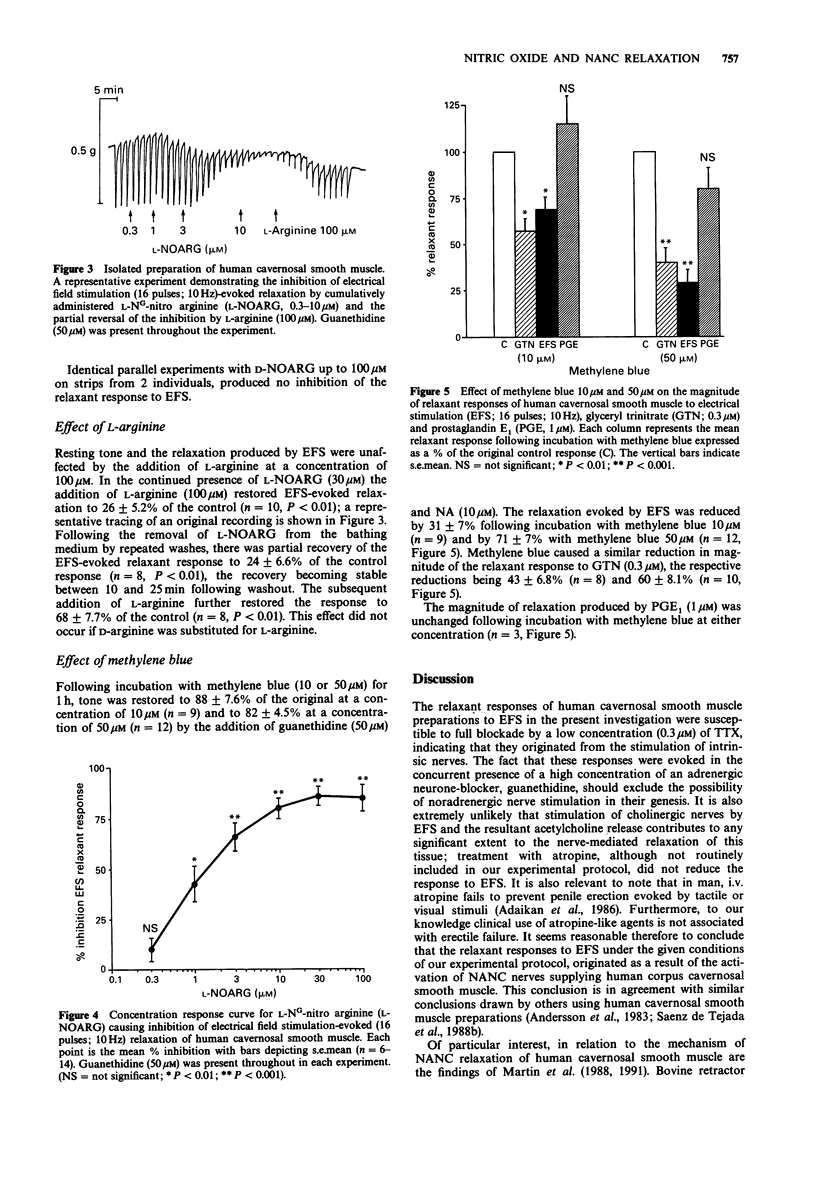
1. The inhibitory transmission in isolated preparations of cavernosal smooth muscle from human penis has been studied. 2. Electrical field stimulation (EFS; 2-64 pulses/train, 0.8 ms pulse duration, 10 Hz) evoked relaxation of preparations treated with guanethidine (50 microM). The EFS-evoked relaxations were atropine-resistant and tetrodotoxin-sensitive indicating their origin to be non-adrenergic, non-cholinergic (NANC) nerve stimulation. 3. EFS-evoked relaxation was attenuated dose-dependently by the nitric oxide (NO)-synthase inhibitor, L-NG-nitro arginine (L-NOARG; 0.3-100 microM) but not by D-NG-nitro arginine. The inhibitory effect of L-NOARG on transmission was antagonized by L-arginine (100 microM), a NO precursor, but not by D-arginine. 4. Incubation with methylene blue (10-50 microM), a known inhibitor of guanylate cyclase activation by NO, caused a concentration-related inhibition of EFS-evoked relaxation. 5. It is concluded that NANC nerve-evoked relaxation of human cavernosal smooth muscle is mediated by NO or a NO-like substance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adaikan P. G., Kottegoda S. R., Ratnam S. S. Is vasoactive intestinal polypeptide the principal transmitter involved in human penile erection? J Urol. 1986 Mar;135(3):638–640. doi: 10.1016/s0022-5347(17)45767-3. [DOI] [PubMed] [Google Scholar]
- Ambache N., Killick S. W., Aboo Aar M. Extraction from ox retractor penis of an inhibitory substance which mimics its atropine-resistant neurogenic relaxation. Br J Pharmacol. 1975 Jul;54(3):409–410. doi: 10.1111/j.1476-5381.1975.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Drummond A. H. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol. 1984 Apr;81(4):665–674. doi: 10.1111/j.1476-5381.1984.tb16133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter C. A., Kadowitz P. J., Ignarro L. J. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can J Physiol Pharmacol. 1981 Feb;59(2):150–156. doi: 10.1139/y81-025. [DOI] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist F., Hedlund H., Andersson K. E. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxation of human isolated corpus cavernosum. Acta Physiol Scand. 1991 Mar;141(3):441–442. doi: 10.1111/j.1748-1716.1991.tb09103.x. [DOI] [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Ignarro L. J., Bush P. A., Buga G. M., Wood K. S., Fukuto J. M., Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990 Jul 31;170(2):843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Ishii K., Chang B., Kerwin J. F., Jr, Huang Z. J., Murad F. N omega-nitro-L-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. Eur J Pharmacol. 1990 Feb 6;176(2):219–223. doi: 10.1016/0014-2999(90)90531-a. [DOI] [PubMed] [Google Scholar]
- Kimura H., Mittal C. K., Murad F. Activation of guanylate cyclase from rat liver and other tissues by sodium azide. J Biol Chem. 1975 Oct 25;250(20):8016–8022. [PubMed] [Google Scholar]
- Klinge E., Sjöstrand N. O. Contraction and relaxation of the retractor penis muscle and the penile artery of the bull. Acta Physiol Scand Suppl. 1974;420:1–88. [PubMed] [Google Scholar]
- Krane R. J., Goldstein I., Saenz de Tejada I. Impotence. N Engl J Med. 1989 Dec 14;321(24):1648–1659. doi: 10.1056/NEJM198912143212406. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Martin W., Smith J. A., Lewis M. J., Henderson A. H. Evidence that inhibitory factor extracted from bovine retractor penis is nitrite, whose acid-activated derivative is stabilized nitric oxide. Br J Pharmacol. 1988 Mar;93(3):579–586. doi: 10.1111/j.1476-5381.1988.tb10313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I., Blanco R., Goldstein I., Azadzoi K., de las Morenas A., Krane R. J., Cohen R. A. Cholinergic neurotransmission in human corpus cavernosum. I. Responses of isolated tissue. Am J Physiol. 1988 Mar;254(3 Pt 2):H459–H467. doi: 10.1152/ajpheart.1988.254.3.H459. [DOI] [PubMed] [Google Scholar]
- Sjöstrand N. O., Eldh J., Samuelson U. E., Alaranta S., Klinge E. The effects of L-arginine and NG-monomethyl L-arginine on the inhibitory neurotransmission of the human corpus cavernosum penis. Acta Physiol Scand. 1990 Oct;140(2):297–298. doi: 10.1111/j.1748-1716.1990.tb09002.x. [DOI] [PubMed] [Google Scholar]
- Tucker J. F., Brave S. R., Charalambous L., Hobbs A. J., Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990 Aug;100(4):663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



