Abstract
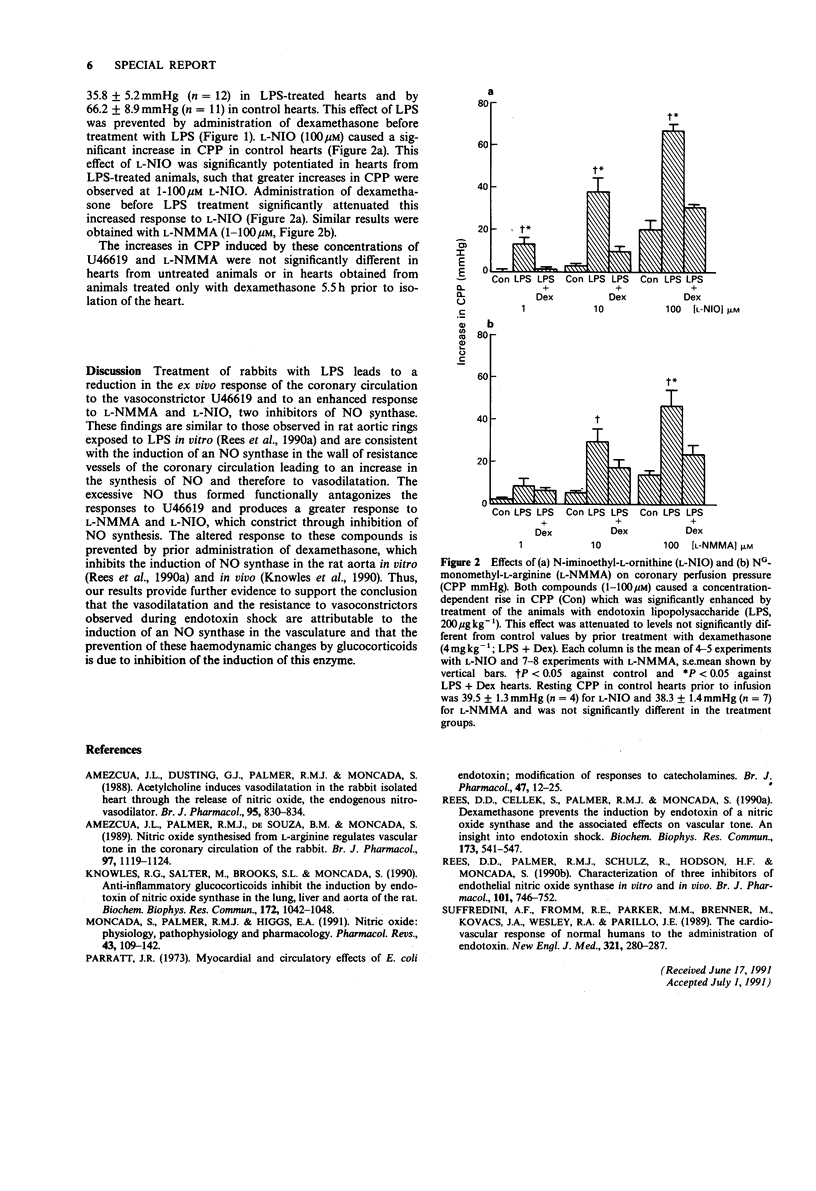
The coronary vasoconstriction induced by the thromboxane mimetic U46619 (9, 11 dideoxy methanoepoxy 9 alpha, 11 alpha prostaglandin F2 alpha, 3-30 nM) was significantly attenuated in hearts obtained from rabbits treated with endotoxin (lipopolysaccharide, LPS, 200 micrograms kg-1, i.v.) 4 h before isolation of the heart. Under these conditions the vasoconstriction induced by two inhibitors of nitric oxide (NO) synthase, NG-monomethyl-L-arginine (L-NMMA) and N-iminoethyl-L-ornithine (L-NIO) (1-100 microM for each) was significantly enhanced when compared to that induced in hearts from control animals. Both the decreased response to U46619 and the increased response to inhibitors of NO synthase were significantly attenuated by administration of dexamethasone (4 mg kg-1, i.v.) 90 min before treatment with LPS. These data are consistent with the induction, by LPS, of an NO synthase, and the inhibition of this induction by dexamethasone. The enhanced NO synthesis contributes to the haemodynamic changes known to occur in endotoxin shock.
Full text
PDF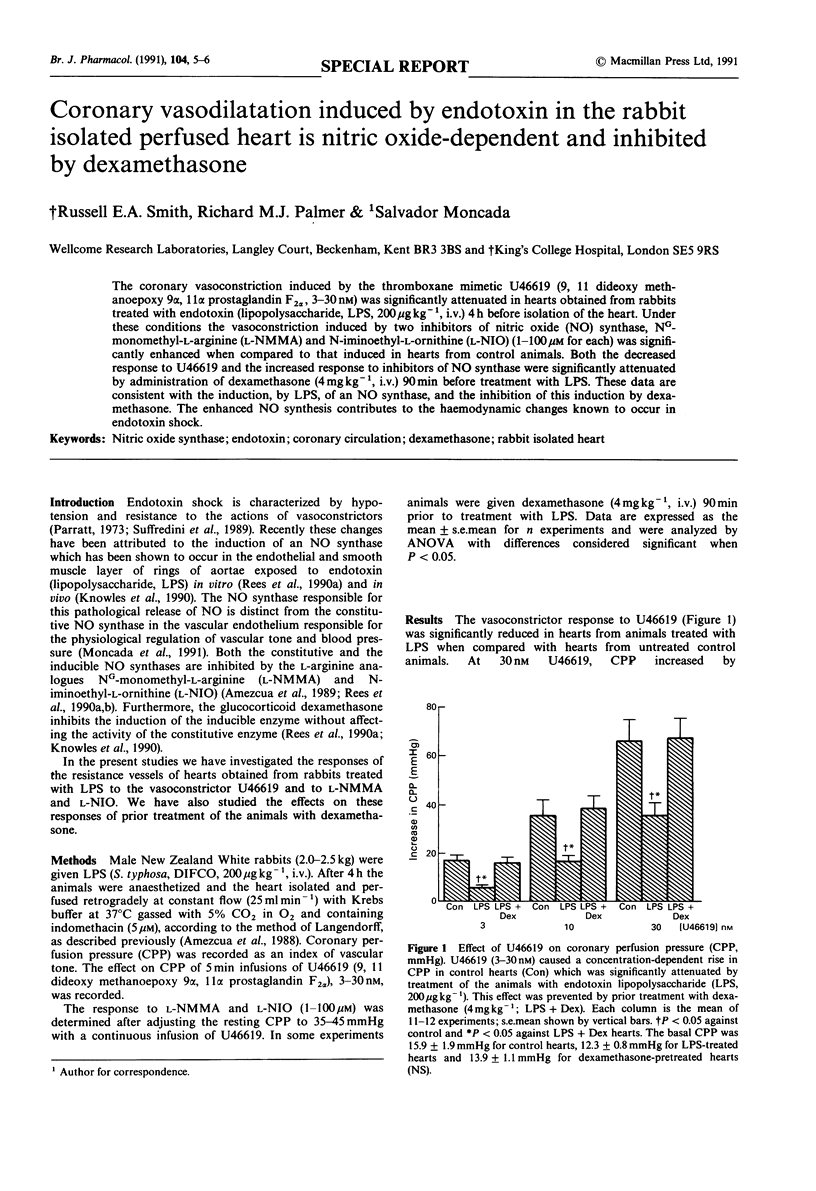
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amezcua J. L., Dusting G. J., Palmer R. M., Moncada S. Acetylcholine induces vasodilatation in the rabbit isolated heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988 Nov;95(3):830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua J. L., Palmer R. M., de Souza B. M., Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989 Aug;97(4):1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Parratt J. R. Myocardial and circulatory effects of E. coli endotoxin; modification of responses to catecholamines. Br J Pharmacol. 1973 Jan;47(1):12–25. doi: 10.1111/j.1476-5381.1973.tb08154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffredini A. F., Fromm R. E., Parker M. M., Brenner M., Kovacs J. A., Wesley R. A., Parrillo J. E. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989 Aug 3;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]



