Abstract
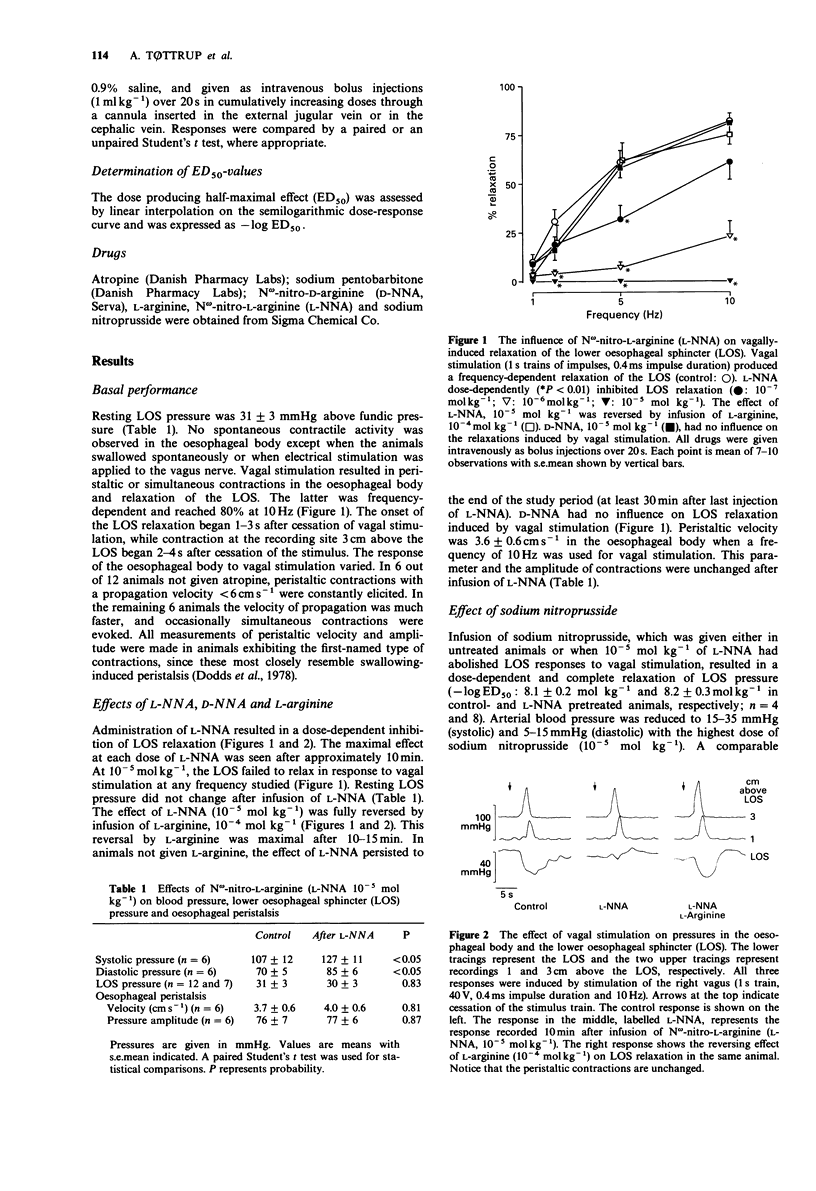
1. The role of the L-arginine-nitric oxide pathway in lower oesophageal sphincter (LOS) relaxation and oesophageal peristalsis was investigated. 2. Twenty four adult opossums were anaesthetized and the right vagus nerve was isolated in the neck and sectioned. Electrical stimulation, applied to the peripheral end of the nerve, resulted in a frequency-dependent relaxation of the LOS, and peristaltic and non-peristaltic contractions in the oesophageal body. 3. N omega-nitro-L-arginine (L-NNA, 10(-8)-10(-5) mol kg-1), an inhibitor of the L-arginine-nitric oxide pathway, inhibited LOS relaxation in a dose-dependent manner, but did not affect resting LOS pressure. At the highest dose of L-NNA no relaxation of the LOS was elicited in response to vagal stimulation. The effect of L-NNA, (10(-5) mol kg-1) was fully reversed by infusion of 10(-4) mol kg-1 L-arginine. Peristaltic velocity and amplitude of contractions in the oesophageal body were unaffected by L-NNA. 4. Infusion of sodium nitroprusside reduced LOS pressure to zero, and the drug was equally potent in control animals (-log ED50:8.1 +/- 0.2 mol kg-1) and in animals pretreated with L-NNA (-log ED50:8.2 +/- 0.3 mol kg-1). This suggests that the effect of L-NNA was not directly on guanylate cyclase. 5. A significant elevation of blood pressure was recorded after administration of L-NNA (10(-5) mol kg-1).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlet A. L. Myogenic peristalsis in isolated preparations of chicken oesophagus. Br J Pharmacol. 1973 May;48(1):36–47. doi: 10.1111/j.1476-5381.1973.tb08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C., Schini V. B., Moncada S., Vanhoutte P. M. Stimulation of cyclic GMP production in cultured endothelial cells of the pig by bradykinin, adenosine diphosphate, calcium ionophore A23187 and nitric oxide. Br J Pharmacol. 1990 Sep;101(1):152–156. doi: 10.1111/j.1476-5381.1990.tb12105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Christensen J., Arthur C., Conklin J. L. Some determinants of latency of off-response to electrical field stimulation in circular layer of smooth muscle of opossum esophagus. Gastroenterology. 1979 Oct;77(4 Pt 1):677–681. [PubMed] [Google Scholar]
- Daniel E. E., Helmy-Elkholy A., Jager L. P., Kannan M. S. Neither a purine nor VIP is the mediator of inhibitory nerves of opossum oesophageal smooth muscle. J Physiol. 1983 Mar;336:243–260. doi: 10.1113/jphysiol.1983.sp014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds W. J., Christensen J., Dent J., Wood J. D., Arndorfer R. C. Esophageal contractions induced by vagal stimulation in the opossum. Am J Physiol. 1978 Oct;235(4):E392–E401. doi: 10.1152/ajpendo.1978.235.4.E392. [DOI] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S. Effects of sodium nitroprusside and verapamil on lower esophageal sphincter. Am J Physiol. 1980 Jan;238(1):G40–G44. doi: 10.1152/ajpgi.1980.238.1.G40. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S. Genesis of basal sphincter pressure: effect of tetrodotoxin on lower esophageal sphincter pressure in opossum in vivo. Gastroenterology. 1976 Jul;71(1):62–67. [PubMed] [Google Scholar]
- Goyal R. K., Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest. 1975 May;55(5):1119–1126. doi: 10.1172/JCI108013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Paterson W. G. Electrical correlates of peristaltic and nonperistaltic contractions in the opossum smooth muscle esophagus. Gastroenterology. 1989 Sep;97(3):665–675. doi: 10.1016/0016-5085(89)90638-0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced relaxation of vascular smooth muscle: role of cyclic GMP. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):281–296. [PubMed] [Google Scholar]
- Rattan S., Gidda J. S., Goyal R. K. Membrane potential and mechanical responses of the opossum esophagus to vagal stimulation and swallowing. Gastroenterology. 1983 Oct;85(4):922–928. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarna S. K., Daniel E. E., Waterfall W. E. Myogenic and neural control systems for esophageal motility. Gastroenterology. 1977 Dec;73(6):1345–1352. [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarbaker D. J., Rattan S., Goyal R. K. Mechanical and electrical activity of esophageal smooth muscle during peristalsis. Am J Physiol. 1984 Feb;246(2 Pt 1):G145–G150. doi: 10.1152/ajpgi.1984.246.2.G145. [DOI] [PubMed] [Google Scholar]
- Torphy T. J., Fine C. F., Burman M., Barnette M. S., Ormsbee H. S., 3rd Lower esophageal sphincter relaxation is associated with increased cyclic nucleotide content. Am J Physiol. 1986 Dec;251(6 Pt 1):G786–G793. doi: 10.1152/ajpgi.1986.251.6.G786. [DOI] [PubMed] [Google Scholar]
- Tucker J. F., Brave S. R., Charalambous L., Hobbs A. J., Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990 Aug;100(4):663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tøttrup A., Svane D., Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Physiol. 1991 Mar;260(3 Pt 1):G385–G389. doi: 10.1152/ajpgi.1991.260.3.G385. [DOI] [PubMed] [Google Scholar]
- Wei E. P., Kontos H. A. H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension. 1990 Aug;16(2):162–169. doi: 10.1161/01.hyp.16.2.162. [DOI] [PubMed] [Google Scholar]
- Weisbrodt N. W., Christensen J. Gradients of contractions in the opossum esophagus. Gastroenterology. 1972 Jun;62(6):1159–1166. [PubMed] [Google Scholar]


