Abstract
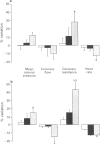
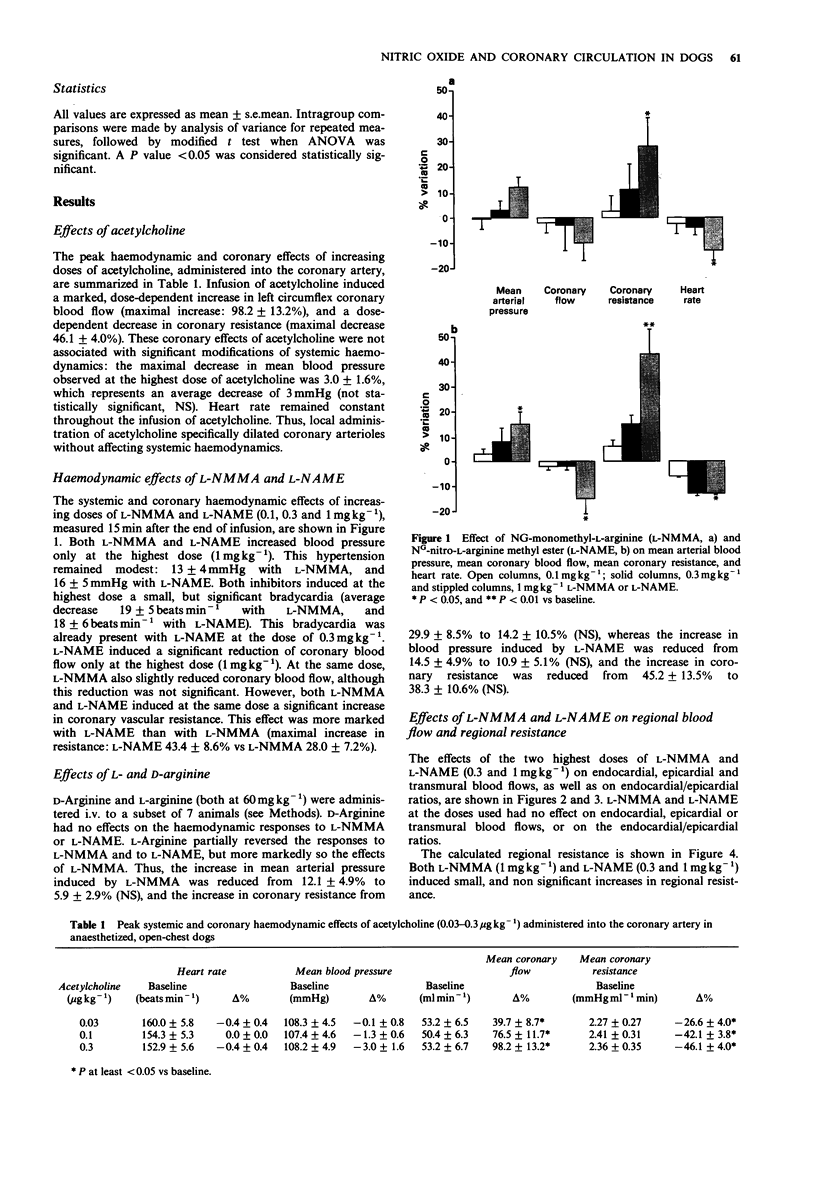
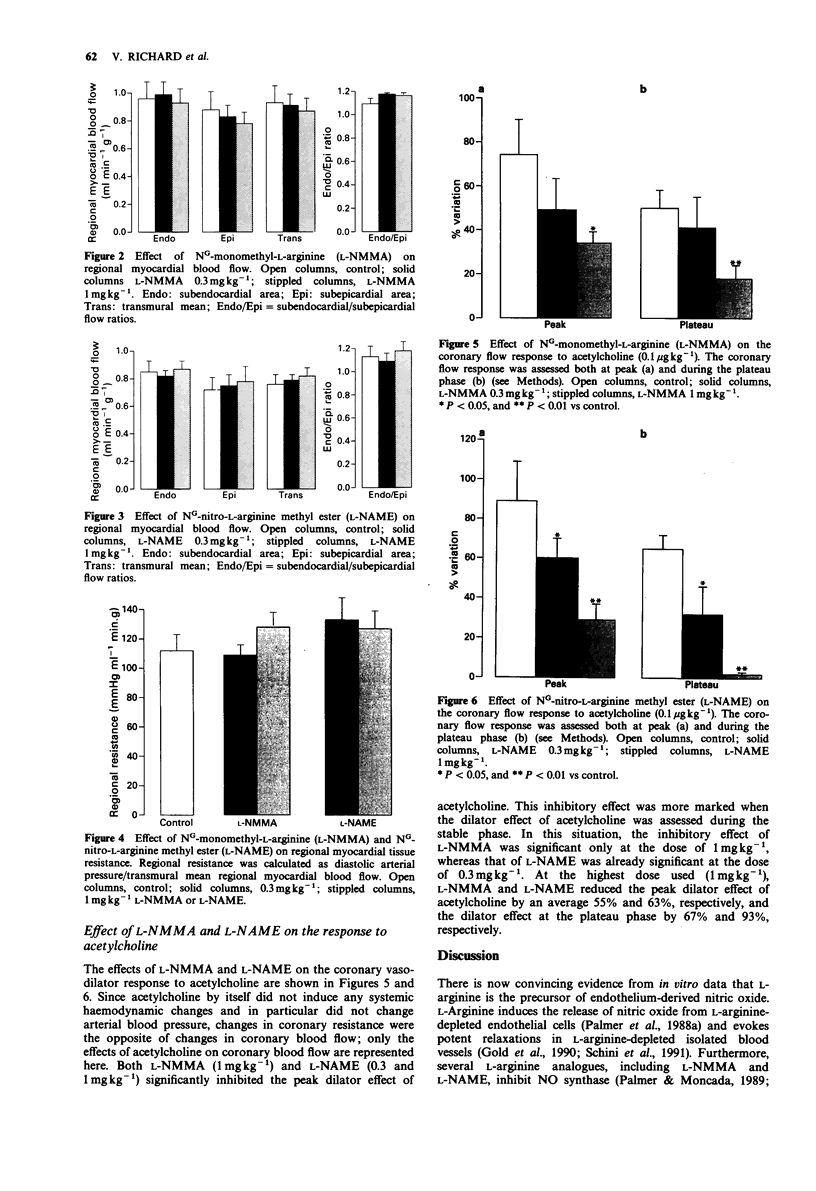
1. The role of endothelial nitric oxide synthesis from L-arginine in the regulation of coronary vascular tone and myocardial tissue perfusion was evaluated in anaesthetized, open-chest dogs. Coronary blood flow was measured with an electromagnetic flow probe placed around the left circumflex coronary artery. Coronary vascular resistance was calculated from mean arterial blood pressure and mean coronary blood flow, whereas regional myocardial tissue flow was determined by use of the radioactive microspheres technique. 2. NG-monomethyl L-arginine (L-NMMA) and NG-nitro-L-arginine methyl ester (L-NAME), administered directly into the left circumflex artery, induced a small increase in arterial blood pressure and an increase in coronary vascular resistance. However, myocardial tissue perfusion, assessed by the microspheres technique (whether subendocardial, subepicardial, or transmural), was unaffected by L-NMMA or L-NAME. 3. Acetylcholine, administered intracoronarily, induced an increase in left circumflex coronary blood flow and a decrease in coronary vascular resistance, without affecting systemic haemodynamics. This coronary vasodilator effect of acetylcholine was markedly inhibited by L-NMMA and L-NAME, the latter being a more potent antagonist than the former. 4. These results indicate that the endothelial L-arginine pathway is largely responsible for the coronary vasodilator effect of acetylcholine. However, although basal release of nitric oxide from L-arginine apparently contributes to the regulation of resting coronary vascular tone, blockade of this pathway does not affect myocardial tissue perfusion, possibly because of compensatory mechanisms occurring at the level of small arterioles and/or capillaries.
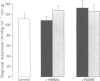
Full text
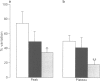
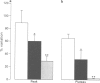
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- Amezcua J. L., Palmer R. M., de Souza B. M., Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989 Aug;97(4):1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilian W. M., Layne S. M., Eastham C. L., Marcus M. L. Heterogeneous microvascular coronary alpha-adrenergic vasoconstriction. Circ Res. 1989 Feb;64(2):376–388. doi: 10.1161/01.res.64.2.376. [DOI] [PubMed] [Google Scholar]
- Chu A., Chambers D. E., Lin C. C., Kuehl W. D., Cobb F. R. Nitric oxide modulates epicardial coronary basal vasomotor tone in awake dogs. Am J Physiol. 1990 Apr;258(4 Pt 2):H1250–H1254. doi: 10.1152/ajpheart.1990.258.4.H1250. [DOI] [PubMed] [Google Scholar]
- Dohi Y., Thiel M. A., Bühler F. R., Lüscher T. F. Activation of endothelial L-arginine pathway in resistance arteries. Effect of age and hypertension. Hypertension. 1990 Aug;16(2):170–179. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1990 Nov;101(3):625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. E., Wood K. S., Byrns R. E., Buga G. M., Ignarro L. J. L-arginine-dependent vascular smooth muscle relaxation and cGMP formation. Am J Physiol. 1990 Dec;259(6 Pt 2):H1813–H1821. doi: 10.1152/ajpheart.1990.259.6.H1813. [DOI] [PubMed] [Google Scholar]
- Hoeffner U., Boulanger C., Vanhoutte P. M. Proximal and distal dog coronary arteries respond differently to basal EDRF but not to NO. Am J Physiol. 1989 Mar;256(3 Pt 2):H828–H831. doi: 10.1152/ajpheart.1989.256.3.H828. [DOI] [PubMed] [Google Scholar]
- Humphries R. G., Carr R. D., Nicol A. K., Tomlinson W., O'Connor S. E. Coronary vasoconstriction in the conscious rabbit following intravenous infusion of L-NG-nitro-arginine. Br J Pharmacol. 1991 Mar;102(3):565–566. doi: 10.1111/j.1476-5381.1991.tb12212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Chang B., Kerwin J. F., Jr, Huang Z. J., Murad F. N omega-nitro-L-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. Eur J Pharmacol. 1990 Feb 6;176(2):219–223. doi: 10.1016/0014-2999(90)90531-a. [DOI] [PubMed] [Google Scholar]
- Kurz M. A., Lamping K. G., Bates J. N., Eastham C. L., Marcus M. L., Harrison D. G. Mechanisms responsible for the heterogeneous coronary microvascular response to nitroglycerin. Circ Res. 1991 Mar;68(3):847–855. doi: 10.1161/01.res.68.3.847. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Hecker M., Vane J. R. The generation of L-arginine in endothelial cells is linked to the release of endothelium-derived relaxing factor. Eur J Pharmacol. 1990 Feb 6;176(2):253–254. doi: 10.1016/0014-2999(90)90541-d. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schini V. B., Vanhoutte P. M. L-arginine evokes both endothelium-dependent and -independent relaxations in L-arginine-depleted aortas of the rat. Circ Res. 1991 Jan;68(1):209–216. doi: 10.1161/01.res.68.1.209. [DOI] [PubMed] [Google Scholar]
- Tschudi M., Richard V., Bühler F. R., Lüscher T. F. Importance of endothelium-derived nitric oxide in porcine coronary resistance arteries. Am J Physiol. 1991 Jan;260(1 Pt 2):H13–H20. doi: 10.1152/ajpheart.1991.260.1.H13. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]