Abstract
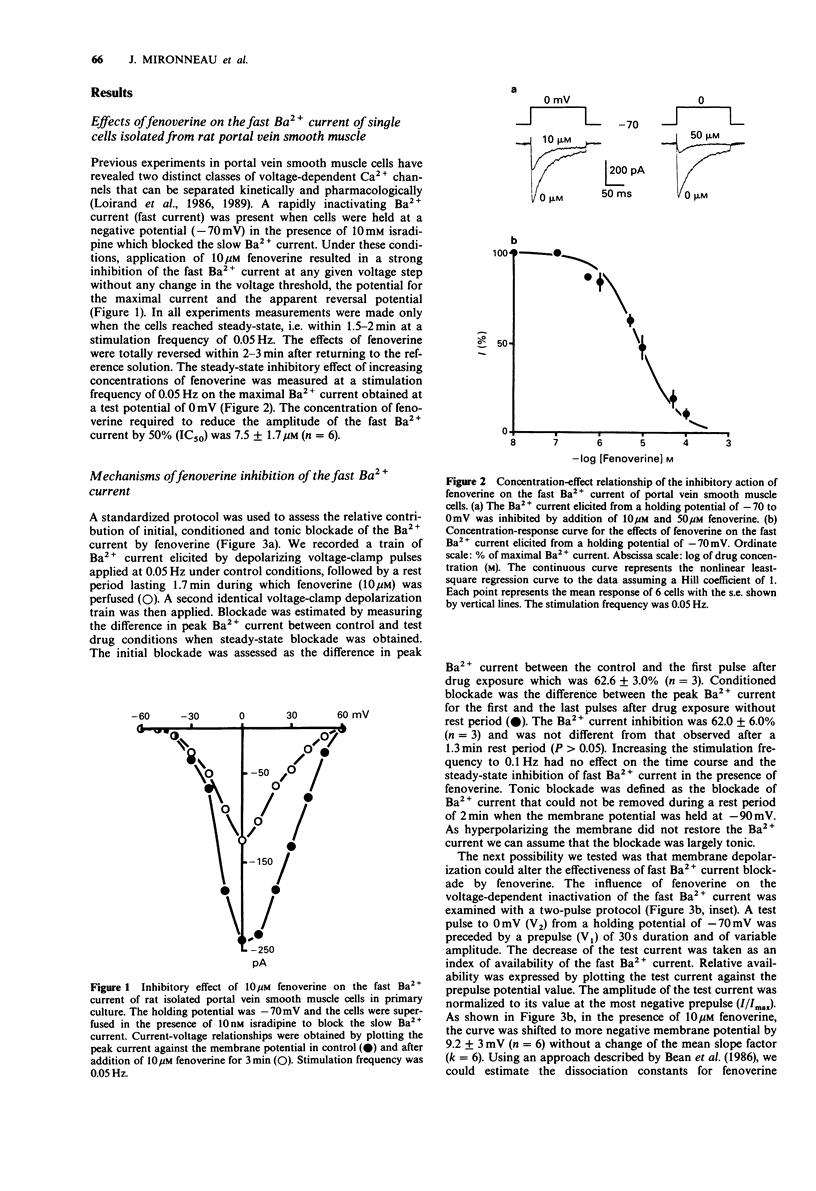
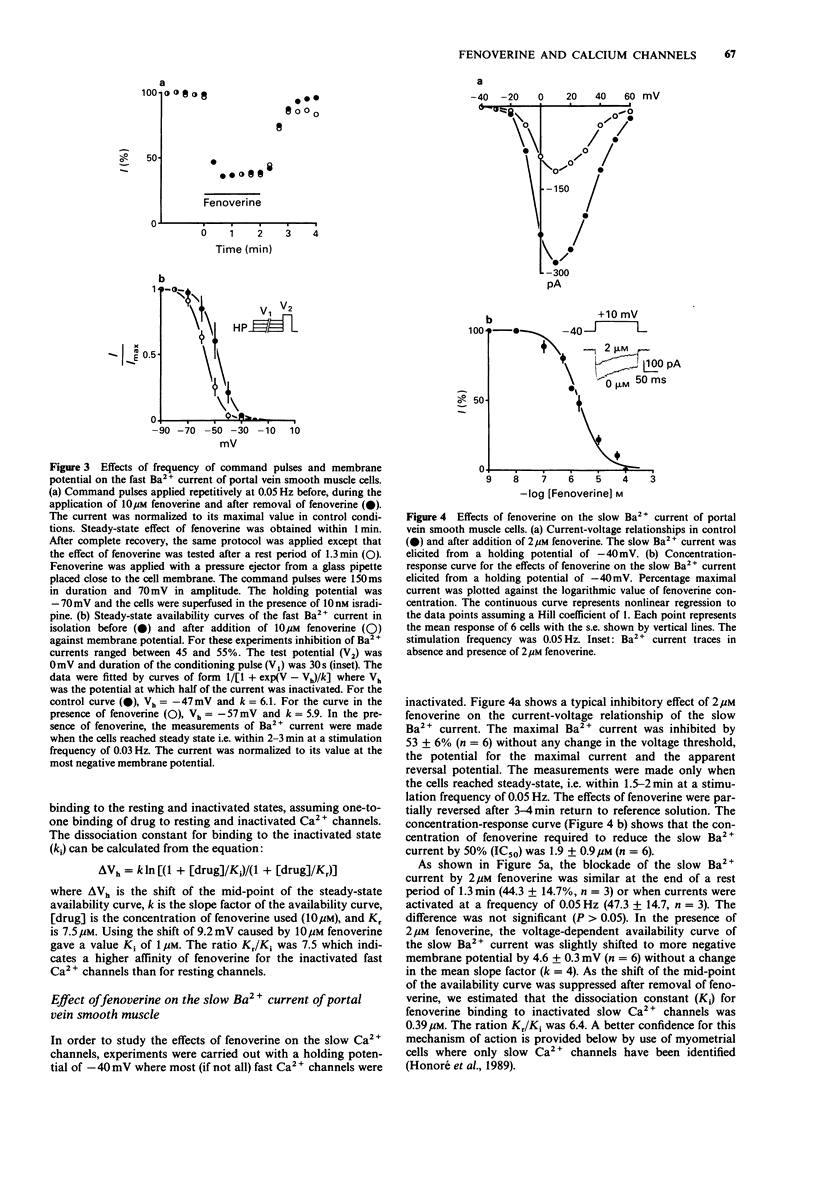
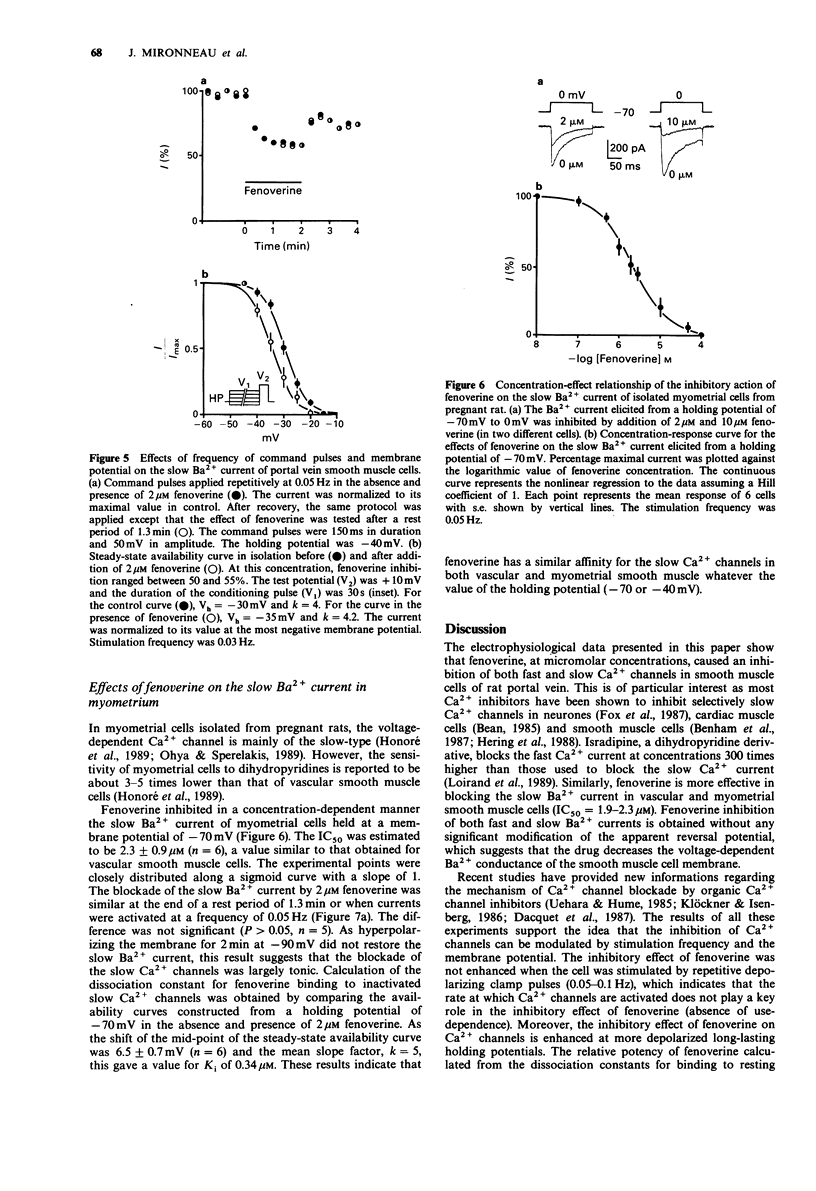
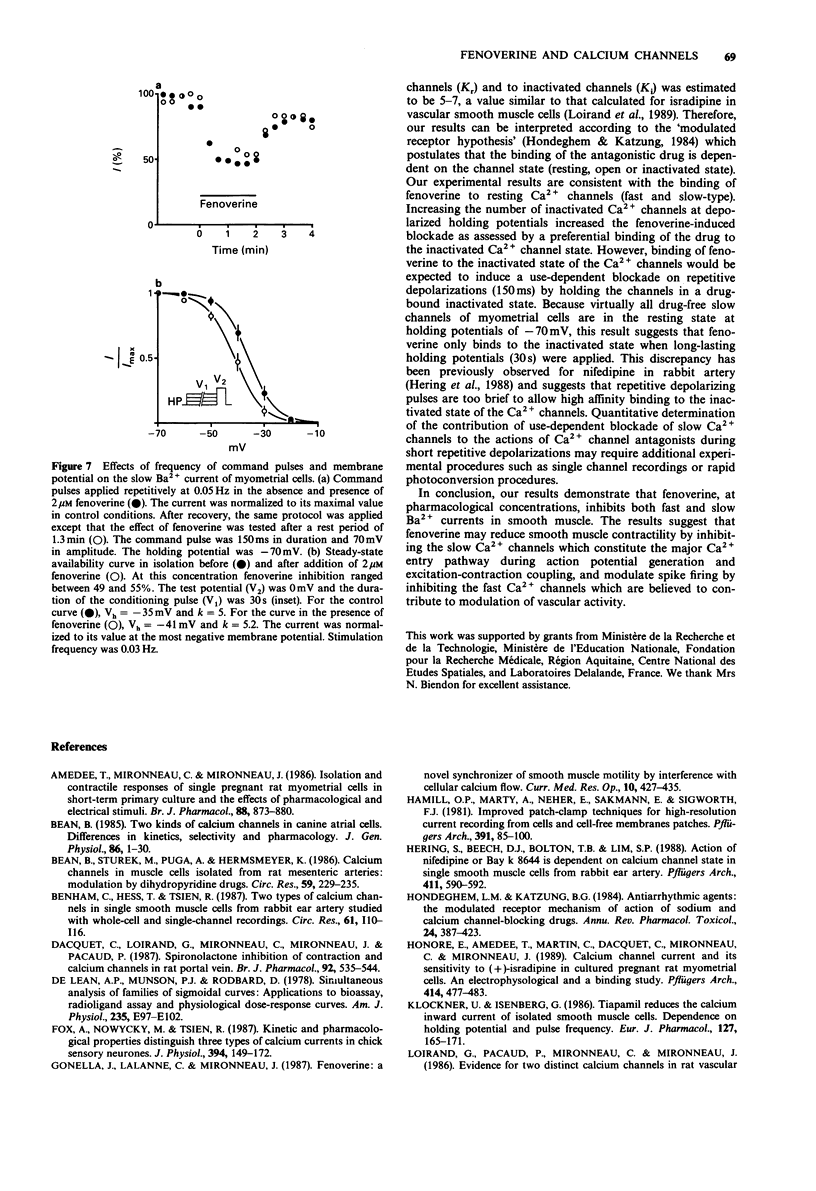
1. The effects of fenoverine, an antispasmodic drug, have been studied on the Ca2+ channel currents of isolated cells from rat portal vein and pregnant myometrium by the patch-clamp technique (whole-cell configuration). 2. Fenoverine inhibited both fast and slow Ca2+ channel currents in a concentration-dependent manner. Half-inhibition of fast Ca2+ channel current (holding potential of -70 mV) and slow Ca2+ channel current (holding potential of -40 mV) in portal vein smooth muscle were obtained at concentrations of 7.5 and 1.9 microM, respectively. In myometrium, the fenoverine concentration which blocked 50% of the slow Ca2+ channel current (holding potential of -70 mV) was 2.3 microM. 3. Administration of fenoverine at rest reduced both Ca2+ channel currents. Currents activated repetitively, at a rate between 0.05 and 0.1 Hz, were inhibited equally which indicates an absence of use-dependent inhibition. 4. When cells held at depolarized membrane potentials at which fast or slow Ca2+ channel currents were strongly inactivated, the inhibitory effects of fenoverine were enhanced on both Ca2+ channel currents which indicates that the fenoverine-induced inhibition was voltage-dependent. The fenoverine concentrations which blocked the inactivated Ca2+ channels were 5-7 times lower than those which blocked the resting Ca2+ channels. 5. Our results show that fenoverine depresses inward currents through fast and slow Ca2+ channels. This effect may be explained by the preferential binding of fenoverine to resting Ca2+ channels. In addition, fenoverine has a higher affinity for inactivated Ca2+ channels than for resting channels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Mironneau C., Mironneau J. Isolation and contractile responses of single pregnant rat myometrial cells in short-term primary culture and the effects of pharmacological and electrical stimuli. Br J Pharmacol. 1986 Aug;88(4):873–880. doi: 10.1111/j.1476-5381.1986.tb16261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacquet C., Loirand G., Mironneau C., Mironneau J., Pacaud P. Spironolactone inhibition of contraction and calcium channels in rat portal vein. Br J Pharmacol. 1987 Nov;92(3):535–544. doi: 10.1111/j.1476-5381.1987.tb11354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonella J., Lalanne C., Mironneau J. Fenoverine: a novel synchronizer of smooth muscle motility by interference with cellular calcium flow. Curr Med Res Opin. 1987;10(7):427–435. doi: 10.1185/03007998709112400. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hering S., Beech D. J., Bolton T. B., Lim S. P. Action of nifedipine or BAY K8644 is dependent on calcium channel state in single smooth muscle cells from rabbit ear artery. Pflugers Arch. 1988 May;411(5):590–592. doi: 10.1007/BF00582383. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423. doi: 10.1146/annurev.pa.24.040184.002131. [DOI] [PubMed] [Google Scholar]
- Honoré E., Amédée T., Martin C., Dacquet C., Mironneau C., Mironneau J. Calcium channel current and its sensitivity to (+) isradipine in cultured pregnant rat myometrial cells. An electrophysiological and a binding study. Pflugers Arch. 1989 Aug;414(4):477–483. doi: 10.1007/BF00585060. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Tiapamil reduces the calcium inward current of isolated smooth muscle cells. Dependence on holding potential and pulse frequency. Eur J Pharmacol. 1986 Aug 15;127(3):165–171. doi: 10.1016/0014-2999(86)90360-2. [DOI] [PubMed] [Google Scholar]
- Loirand G., Mironneau C., Mironneau J., Pacaud P. Two types of calcium currents in single smooth muscle cells from rat portal vein. J Physiol. 1989 May;412:333–349. doi: 10.1113/jphysiol.1989.sp017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Mironneau C., Mironneau J. Evidence for two distinct calcium channels in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1986 Nov;407(5):566–568. doi: 10.1007/BF00657519. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Sperelakis N. Fast Na+ and slow Ca2+ channels in single uterine muscle cells from pregnant rats. Am J Physiol. 1989 Aug;257(2 Pt 1):C408–C412. doi: 10.1152/ajpcell.1989.257.2.C408. [DOI] [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]


