Abstract
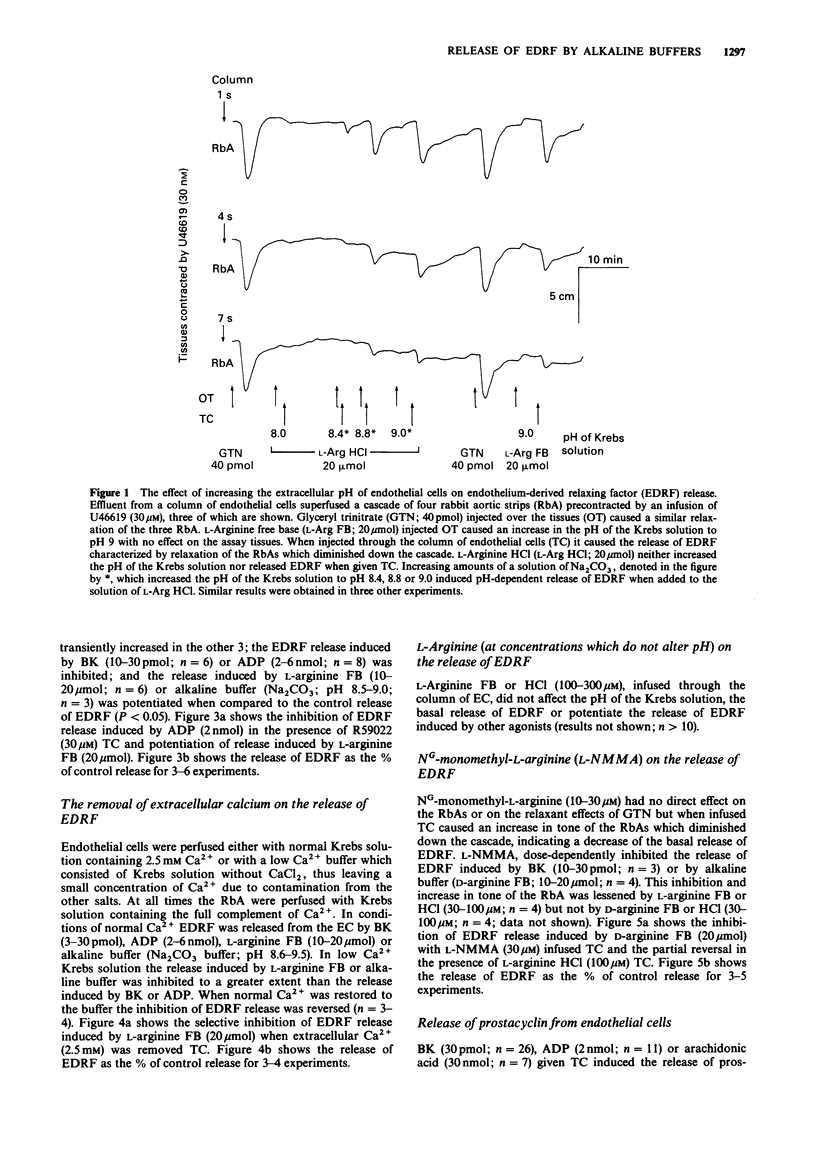
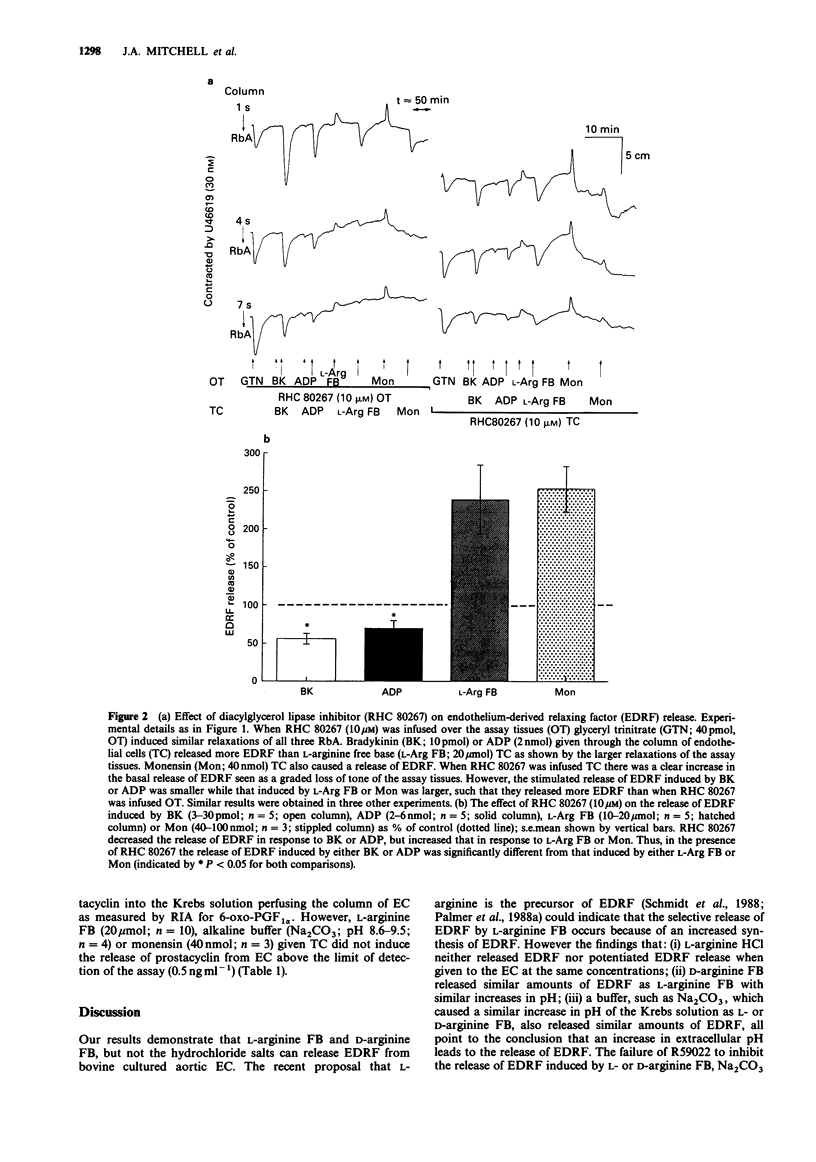
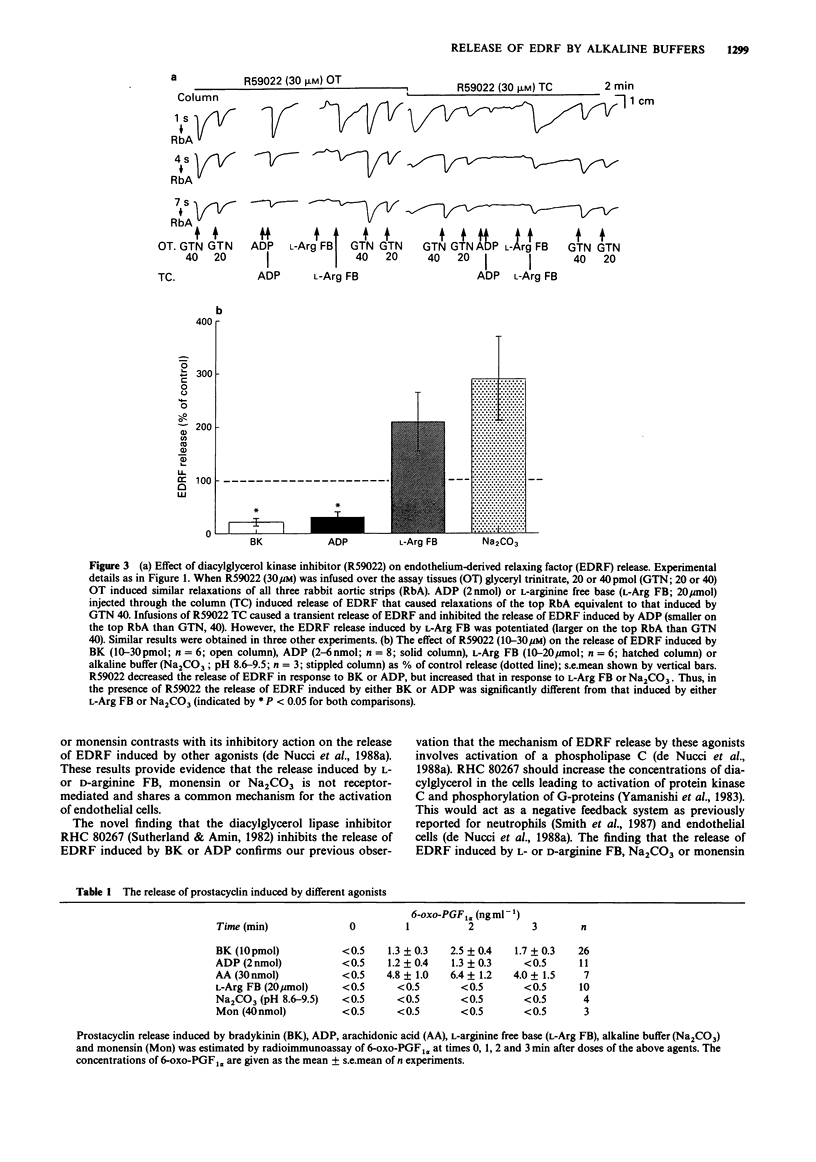
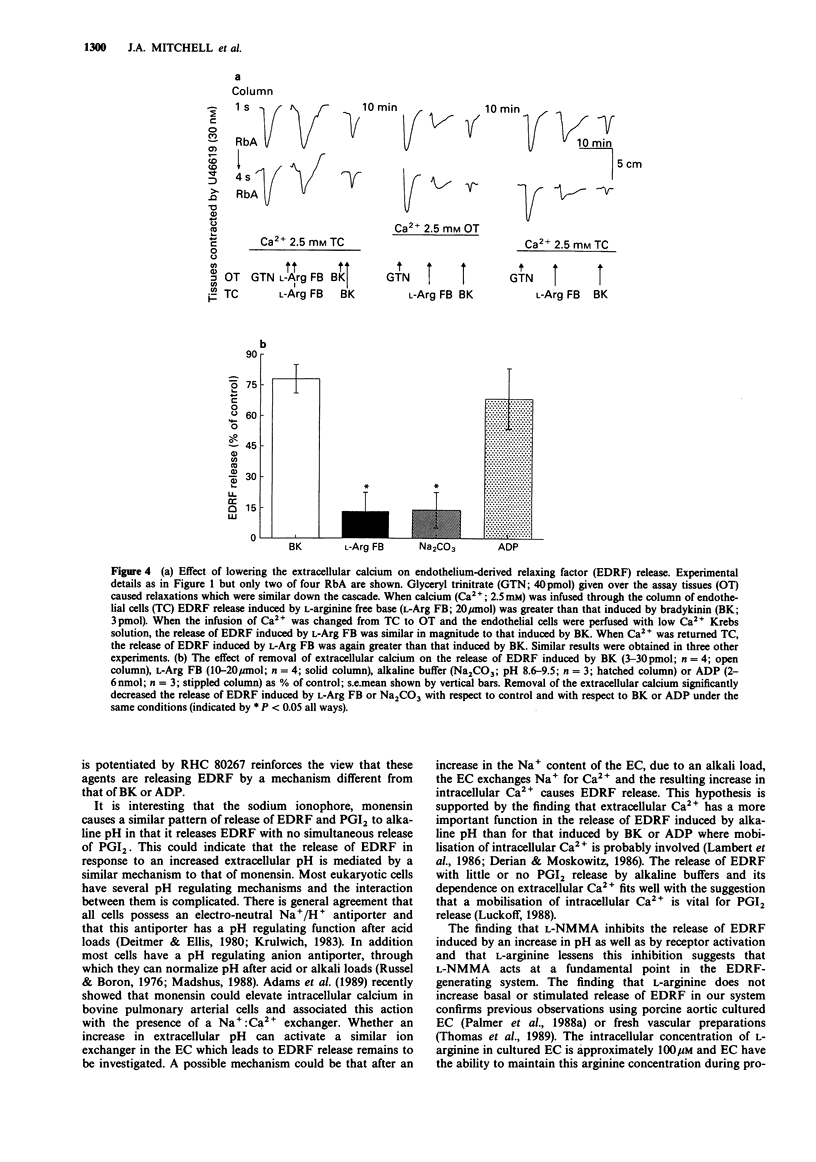
1. Release of endothelium-derived relaxing factor (EDRF) and prostacyclin (PGI2) from bovine cultured aortic endothelial cells (EC) was measured by bioassay and radioimmunoassay, respectively. 2. Bradykinin (BK, 3-30 pmol), adenosine diphosphate (ADP, 2-6 nmol) or the sodium ionophore monensin (40-100 nmol) injected through a column of EC released EDRF. L-Arginine free base (FB; 10-20 mumol) or D-arginine FB (10-20 mumol) injected through the column of EC released similar amounts of EDRF and also caused an increase in pH of the Krebs solution perfusing the EC from 7.5-8.0 to 8.6-9.5. Sodium carbonate (Na2CO3) an alkaline buffer which caused the same changes in the pH of the Krebs solution also induced the same release of EDRF. The hydrochloride salts of L- or D-arginine did not cause either release of EDRF when injected through the column of EC or increases in the pH of the Krebs solution. 3. Inhibitors of either diacylglycerol lipase (RHC 80267) or kinase (R59022) inhibited the release of EDRF induced by BK or ADP but potentiated the release induced by L-arginine FB, monensin (40-100 nmol) or alkaline buffer (Na2CO3). R59022 and RHC 80267 infused through the EC increased the basal release of EDRF. 4. When calcium chloride was omitted from the Krebs solution the release of EDRF induced by alkaline buffer (Na2CO3; pH 8.6-9.5) or L-arginine FB (10-20 mumol) was selectively inhibited when compared to that induced by BK (3-30 pmol) or ADP (2-6 nmol). This inhibition was reversed when calcium (2.5 mM) was restored. 5. NG-monomethyl-L-arginine (NMMA; 30 microM) inhibited release of EDRF induced by BK (10-30 pmol) or alkaline buffers (Na2CO3 or D-arginine FB; pH 8.6-9.5). This inhibition was partially reversed by L- but not D-arginine FB or HCl (30-100 microM). 6. Prostacyclin was released when BK (10 pmol), ADP (2 nmol) or arachidonic acid (30 nmol) were injected through the column of EC. However, monensin (40 nmol) or alkaline buffers (pH 8.6-9.5) did not release detectable amounts of PGI2 as measured by radioimmunoassay for 6-oxo-prostaglandin F1 alpha.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deitmer J. W., Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980 Jul;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Förstermann U., Goppelt-Strübe M., Frölich J. C., Busse R. Inhibitors of acyl-coenzyme A:lysolecithin acyltransferase activate the production of endothelium-derived vascular relaxing factor. J Pharmacol Exp Ther. 1986 Jul;238(1):352–359. [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Madshus I. H. Regulation of intracellular pH in eukaryotic cells. Biochem J. 1988 Feb 15;250(1):1–8. doi: 10.1042/bj2500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Hecker M., Vane J. R. The generation of L-arginine in endothelial cells is linked to the release of endothelium-derived relaxing factor. Eur J Pharmacol. 1990 Feb 6;176(2):253–254. doi: 10.1016/0014-2999(90)90541-d. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by endothelium-derived relaxing factor from cultured endothelial cells. Eur J Pharmacol. 1987 Mar 17;135(2):247–250. doi: 10.1016/0014-2999(87)90620-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Uhing R. J., Snyderman R. Nucleotide regulatory protein-mediated activation of phospholipase C in human polymorphonuclear leukocytes is disrupted by phorbol esters. J Biol Chem. 1987 May 5;262(13):6121–6127. [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C. A., Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982 Dec 10;257(23):14006–14010. [PubMed] [Google Scholar]
- Thomas G., Hecker M., Ramwell P. W. Vascular activity of polycations and basic amino acids: L-arginine does not specifically elicit endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Jan 16;158(1):177–180. doi: 10.1016/s0006-291x(89)80194-9. [DOI] [PubMed] [Google Scholar]
- VANE J. R. THE USE OF ISOLATED ORGANS FOR DETECTING ACTIVE SUBSTANCES IN THE CIRCULATING BLOOD. Br J Pharmacol Chemother. 1964 Oct;23:360–373. doi: 10.1111/j.1476-5381.1964.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R. Prostaglandin research. Eicosanoids. 1988;1(1):1–2. [PubMed] [Google Scholar]
- Yamanishi J., Takai Y., Kaibuchi K., Sano K., Castagna M., Nishizuka Y. Synergistic functions of phorbol ester and calcium in serotonin release from human platelets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):778–786. doi: 10.1016/0006-291x(83)91529-2. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D. C., Roevens P., Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985 Dec 15;260(29):15762–15770. [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]



