Abstract
1. Xenopus oocytes were injected with various muscle and neuronal nicotinic acetylcholine receptor (ACh receptor, cholinoceptor) subunit RNA combinations and their pharmacological properties studied using two-electrode voltage clamp. The functional expression of one of these combinations, rat alpha 4-2 beta 2, has not been previously described. The alpha 4-2 mRNA is a splicing variant transcribed from the alpha 4 gene. In the experiments reported here, the alpha 4-2 beta 2 subtype was functionally indistinguishable from the alpha 4-1 beta 2 subtype. 2. For each subtype, the relative potency of nicotine compared with acetylcholine was obtained by estimating the relative concentration of nicotine which would elicit the same current response as 0.1 microM Ach. The ratios of these concentrations (nicotine: ACh) for the mouse muscle ACh receptor-(alpha 1 beta 1 gamma delta) was 96.1:1. In contrast, the ratios for the rat neuronal subtypes were: alpha 2 beta 2, 1.01:1; alpha 3 beta 2, 2.01:1; alpha 4 beta 2, 0.76:1 and alpha 4-2 beta 2, 0.76:1. The much greater relative nicotine sensitivity of the neuronal subtypes as compared with muscle receptors illustrates their potential to mediate the psychoactive and addictive effects of nicotine. However, it does not appear that the differences in relative nicotinic sensitivity among the neuronal receptors themselves can be used as a simple discriminative tool in neuronal tissue. 3. The slopes of the log dose-log response curves at low ACh concentrations were all greater than 1 but less than 2, suggesting that at least two agonist binding sites mediate the functional response of each hetero-oligomer. 4. The response of all the neuronal subtypes to ACh could be inhibited by the psychoactive drugs mecamylamine, amitriptyline, phencyclidine, trifluoperazine and promethazine. With the exception of the very potent antagonist, mecamylamine, the degree of block of the peak current to ACh produced by 10 microM concentrations of these drugs was remarkably similar (around 50%). 5. The degree of inhibition produced when the antipsychotic drug, trifluoperazine, was co-applied with ACh increased as the duration of application increased. Such an effect was not observed with promethazine, a related phenothiazine derivative which does not have antipsychotic actions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Tsai M. C., Aronstam R. S., Eldefrawi A. T., Eldefrawi M. E. Sites of action of phencyclidine. II. Interaction with the ionic channel of the nicotinic receptor. Mol Pharmacol. 1980 Sep;18(2):167–178. [PubMed] [Google Scholar]
- Amador M., Dani J. A. MK-801 inhibition of nicotinic acetylcholine receptor channels. Synapse. 1991 Mar;7(3):207–215. doi: 10.1002/syn.890070305. [DOI] [PubMed] [Google Scholar]
- Anand R., Lindstrom J. Nucleotide sequence of the human nicotinic acetylcholine receptor beta 2 subunit gene. Nucleic Acids Res. 1990 Jul 25;18(14):4272–4272. doi: 10.1093/nar/18.14.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballivet M., Nef P., Couturier S., Rungger D., Bader C. R., Bertrand D., Cooper E. Electrophysiology of a chick neuronal nicotinic acetylcholine receptor expressed in Xenopus oocytes after cDNA injection. Neuron. 1988 Nov;1(9):847–852. doi: 10.1016/0896-6273(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Baron J. A. Cigarette smoking and Parkinson's disease. Neurology. 1986 Nov;36(11):1490–1496. doi: 10.1212/wnl.36.11.1490. [DOI] [PubMed] [Google Scholar]
- Benowitz N. L. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988 Nov 17;319(20):1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Ballivet M., Rungger D. Activation and blocking of neuronal nicotinic acetylcholine receptor reconstituted in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1993–1997. doi: 10.1073/pnas.87.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Connolly J., Deneris E., Goldman D., Heinemann S., Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Boulter J., Evans K., Martin G., Mason P., Stengelin S., Goldman D., Heinemann S., Patrick J. Isolation and sequence of cDNA clones coding for the precursor to the gamma subunit of mouse muscle nicotinic acetylcholine receptor. J Neurosci Res. 1986;16(1):37–49. doi: 10.1002/jnr.490160106. [DOI] [PubMed] [Google Scholar]
- Boulter J., Luyten W., Evans K., Mason P., Ballivet M., Goldman D., Stengelin S., Martin G., Heinemann S., Patrick J. Isolation of a clone coding for the alpha-subunit of a mouse acetylcholine receptor. J Neurosci. 1985 Sep;5(9):2545–2552. doi: 10.1523/JNEUROSCI.05-09-02545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., O'Shea-Greenfield A., Duvoisin R. M., Connolly J. G., Wada E., Jensen A., Gardner P. D., Ballivet M., Deneris E. S., McKinnon D. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990 Mar 15;265(8):4472–4482. [PubMed] [Google Scholar]
- Brazell M. P., Mitchell S. N., Joseph M. H., Gray J. A. Acute administration of nicotine increases the in vivo extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid and ascorbic acid preferentially in the nucleus accumbens of the rat: comparison with caudate-putamen. Neuropharmacology. 1990 Dec;29(12):1177–1185. doi: 10.1016/0028-3908(90)90042-p. [DOI] [PubMed] [Google Scholar]
- Buller A. L., White M. M. Functional acetylcholine receptors expressed in Xenopus oocytes after injection of Torpedo beta, gamma, and delta subunit RNAs are a consequence of endogenous oocyte gene expression. Mol Pharmacol. 1990 Mar;37(3):423–428. [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES R. M. The excitation of Renshaw cells by pharmacological agents applied electrophoretically. J Physiol. 1958 May 28;141(3):435–445. doi: 10.1113/jphysiol.1958.sp005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Pinset C., Ribera A. B. Effects of chlorpromazine and phencyclidine on mouse C2 acetylcholine receptor kinetics. J Physiol. 1986 Sep;378:497–513. doi: 10.1113/jphysiol.1986.sp016232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. B., Schwartz R. D., Paul S. M., Pert C. B., Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985 May;5(5):1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M. S., Littleton J. M. Lack of stereoselectivity in ability of nicotine to release dopamine from rat synaptosomal preparations. J Neurochem. 1983 Nov;41(5):1297–1302. doi: 10.1111/j.1471-4159.1983.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Connolly J. G. Structure-function relationships in nicotinic acetylcholine receptors. Comp Biochem Physiol A Comp Physiol. 1989;93(1):221–231. doi: 10.1016/0300-9629(89)90210-7. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Dunn S. M., Barnard E. A., Dolly J. O., Lai F. A., Ray N., Raftery M. A. Brain and muscle nicotinic acetylcholine receptors are different but homologous proteins. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5208–5212. doi: 10.1073/pnas.82.15.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Couturier S., Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991 Mar 21;350(6315):235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Couturier S., Bertrand D., Matter J. M., Hernandez M. C., Bertrand S., Millar N., Valera S., Barkas T., Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990 Dec;5(6):847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Couturier S., Erkman L., Valera S., Rungger D., Bertrand S., Boulter J., Ballivet M., Bertrand D. Alpha 5, alpha 3, and non-alpha 3. Three clustered avian genes encoding neuronal nicotinic acetylcholine receptor-related subunits. J Biol Chem. 1990 Oct 15;265(29):17560–17567. [PubMed] [Google Scholar]
- Creese I., Burt D. R., Snyder S. H. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976 Apr 30;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Deneris E. S., Connolly J., Boulter J., Wada E., Wada K., Swanson L. W., Patrick J., Heinemann S. Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron. 1988 Mar;1(1):45–54. doi: 10.1016/0896-6273(88)90208-5. [DOI] [PubMed] [Google Scholar]
- Deneris E. S., Connolly J., Rogers S. W., Duvoisin R. Pharmacological and functional diversity of neuronal nicotinic acetylcholine receptors. Trends Pharmacol Sci. 1991 Jan;12(1):34–40. doi: 10.1016/0165-6147(91)90486-c. [DOI] [PubMed] [Google Scholar]
- Dilger J. P., Brett R. S. Direct measurement of the concentration- and time-dependent open probability of the nicotinic acetylcholine receptor channel. Biophys J. 1990 Apr;57(4):723–731. doi: 10.1016/S0006-3495(90)82593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasari D., Chini B., Tarroni P., Clementi F. Molecular cloning of human neuronal nicotinic receptor alpha 3-subunit. Neurosci Lett. 1990 Apr 6;111(3):351–356. doi: 10.1016/0304-3940(90)90287-j. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Steep concentration dependence and fast desensitization of nicotinic channel currents elicited by acetylcholine pulses, studied in adult vertebrate muscle. Pflugers Arch. 1991 Jan;417(5):509–516. doi: 10.1007/BF00370947. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Chang J. Y., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: serine-262 of the delta subunit is labeled by [3H]chlorpromazine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2719–2723. doi: 10.1073/pnas.83.8.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the beta and delta chains. Biochemistry. 1987 May 5;26(9):2410–2418. doi: 10.1021/bi00383a003. [DOI] [PubMed] [Google Scholar]
- Goldman D., Deneris E., Luyten W., Kochhar A., Patrick J., Heinemann S. Members of a nicotinic acetylcholine receptor gene family are expressed in different regions of the mammalian central nervous system. Cell. 1987 Mar 27;48(6):965–973. doi: 10.1016/0092-8674(87)90705-7. [DOI] [PubMed] [Google Scholar]
- Halliwell R. F., Peters J. A., Lambert J. J. The mechanism of action and pharmacological specificity of the anticonvulsant NMDA antagonist MK-801: a voltage clamp study on neuronal cells in culture. Br J Pharmacol. 1989 Feb;96(2):480–494. doi: 10.1111/j.1476-5381.1989.tb11841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman D. S., Claudio T. Coexpression of two distinct muscle acetylcholine receptor alpha-subunits during development. Nature. 1990 Jan 25;343(6256):372–375. doi: 10.1038/343372a0. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Oswald R. E., Changeux J. P. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
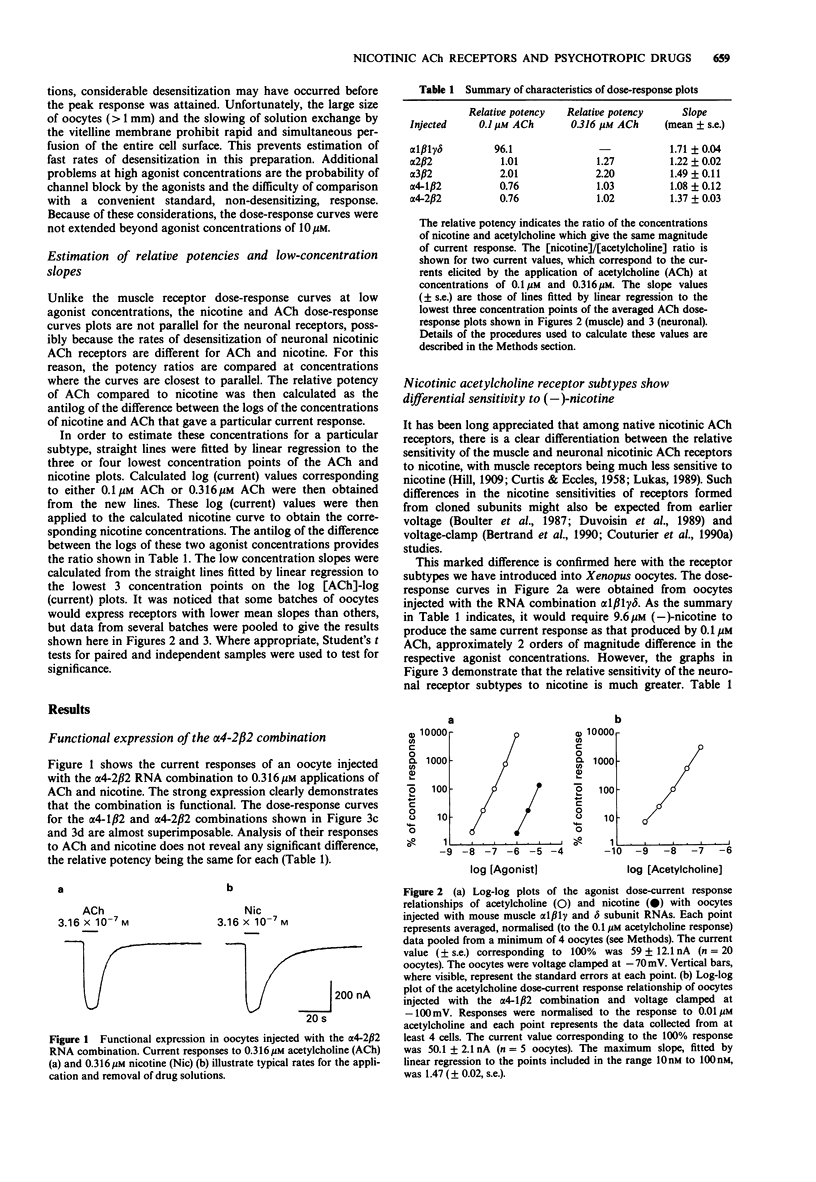
- Hill A. V. The mode of action of nicotine and curari, determined by the form of the contraction curve and the method of temperature coefficients. J Physiol. 1909 Dec 23;39(5):361–373. doi: 10.1113/jphysiol.1909.sp001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Tester B. A., Weber E. Interaction of MK-801 with the nicotinic acetylcholine receptor-associated ion channel from electroplax. Eur J Pharmacol. 1989 May 19;164(2):397–398. doi: 10.1016/0014-2999(89)90487-1. [DOI] [PubMed] [Google Scholar]
- Kurosaki T., Fukuda K., Konno T., Mori Y., Tanaka K., Mishina M., Numa S. Functional properties of nicotinic acetylcholine receptor subunits expressed in various combinations. FEBS Lett. 1987 Apr 20;214(2):253–258. doi: 10.1016/0014-5793(87)80065-0. [DOI] [PubMed] [Google Scholar]
- Luetje C. W., Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991 Mar;11(3):837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas R. J. Pharmacological distinctions between functional nicotinic acetylcholine receptors on the PC12 rat pheochromocytoma and the TE671 human medulloblastoma. J Pharmacol Exp Ther. 1989 Oct;251(1):175–182. [PubMed] [Google Scholar]
- Luther M. A., Schoepfer R., Whiting P., Casey B., Blatt Y., Montal M. S., Montal M., Linstrom J. A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci. 1989 Mar;9(3):1082–1096. doi: 10.1523/JNEUROSCI.09-03-01082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
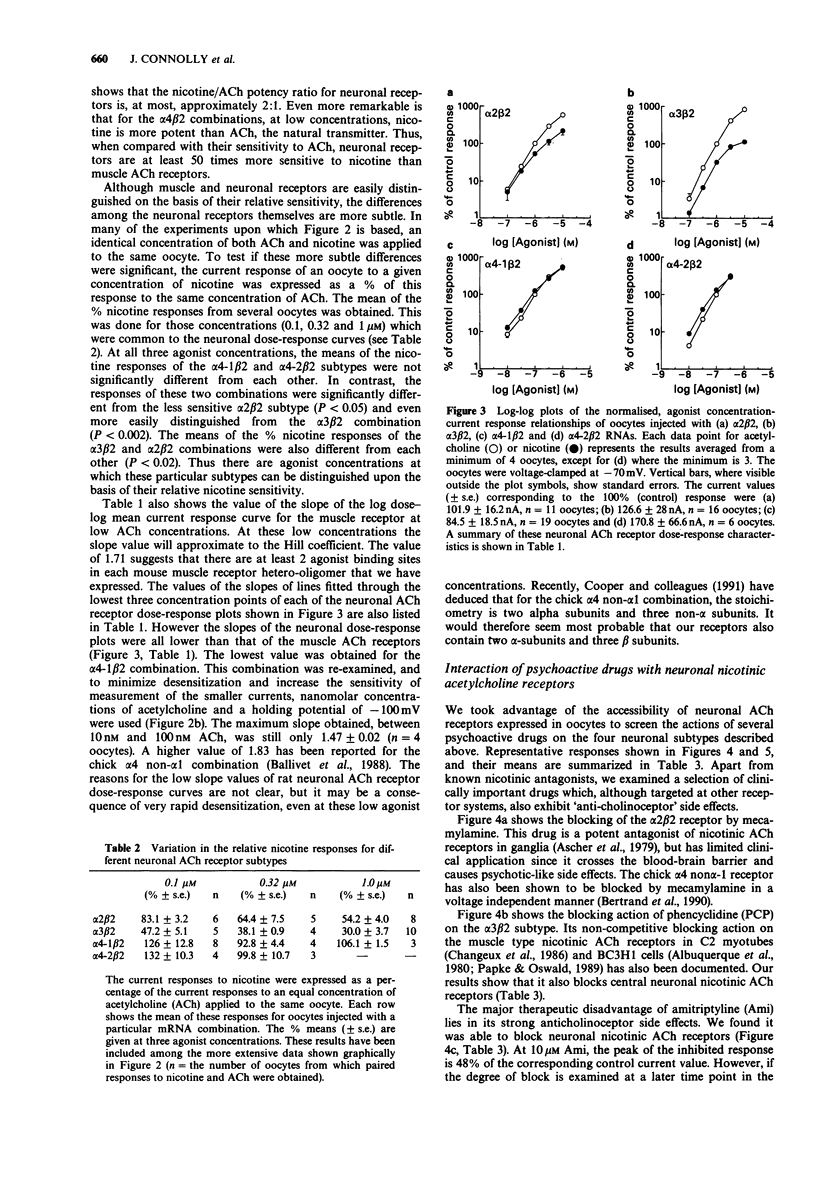
- MacDonald J. F., Miljkovic Z., Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987 Aug;58(2):251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. E., Manderscheid P. Z., Montgomery S. P., Norman A. B., Sanberg P. R. Nicotine and cannabinoids as adjuncts to neuroleptics in the treatment of Tourette syndrome and other motor disorders. Life Sci. 1989;44(21):1521–1525. doi: 10.1016/0024-3205(89)90444-x. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Christensen B. N. Mecamylamine is a selective non-competitive antagonist of N-methyl-D-aspartate- and aspartate-induced currents in horizontal cells dissociated from the catfish retina. Neurosci Lett. 1988 Nov 22;94(1-2):93–98. doi: 10.1016/0304-3940(88)90276-5. [DOI] [PubMed] [Google Scholar]
- Papke R. L., Boulter J., Patrick J., Heinemann S. Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron. 1989 Nov;3(5):589–596. doi: 10.1016/0896-6273(89)90269-9. [DOI] [PubMed] [Google Scholar]
- Papke R. L., Heinemann S. F. The role of the beta 4-subunit in determining the kinetic properties of rat neuronal nicotinic acetylcholine alpha 3-receptors. J Physiol. 1991;440:95–112. doi: 10.1113/jphysiol.1991.sp018698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R. L., Oswald R. E. Mechanisms of noncompetitive inhibition of acetylcholine-induced single-channel currents. J Gen Physiol. 1989 May;93(5):785–811. doi: 10.1085/jgp.93.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. A., Malone H. M., Lambert J. J. Antagonism of 5-HT3 receptor mediated currents in murine N1E-115 neuroblastoma cells by (+)-tubocurarine. Neurosci Lett. 1990 Mar 2;110(1-2):107–112. doi: 10.1016/0304-3940(90)90796-c. [DOI] [PubMed] [Google Scholar]
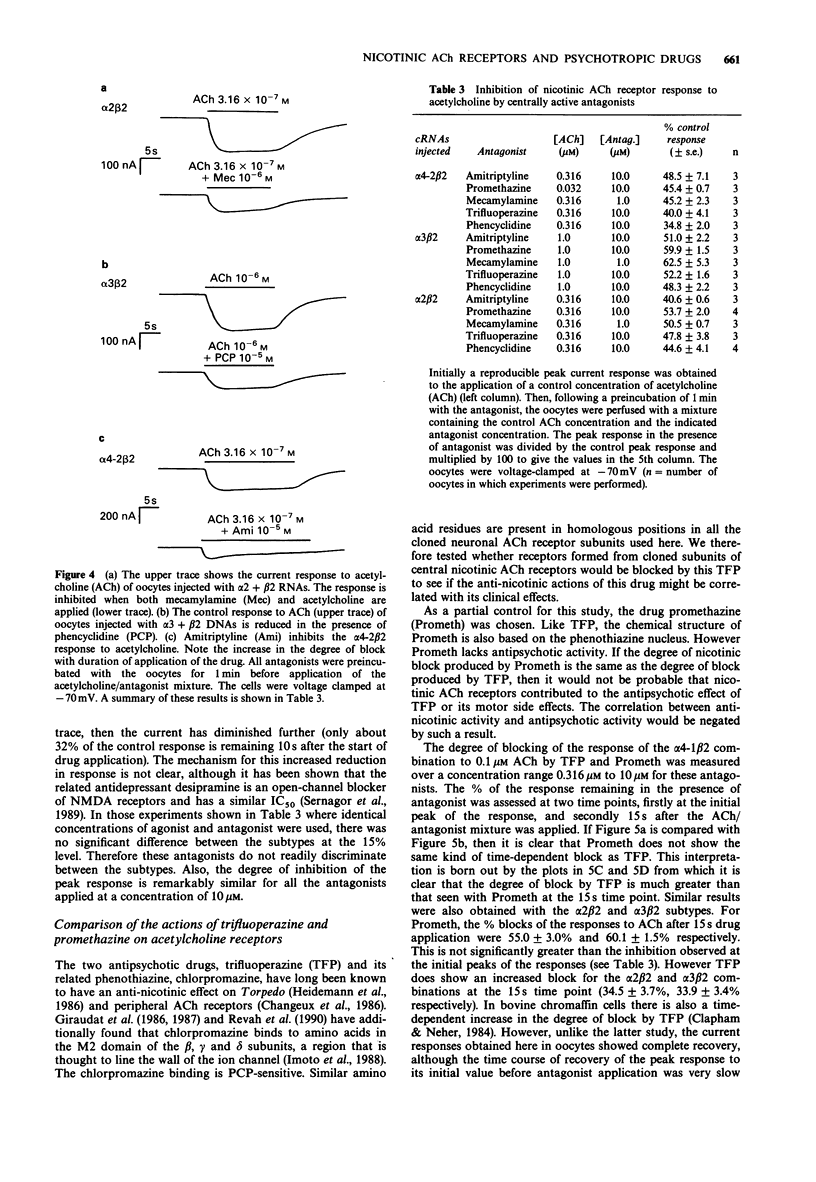
- Rapier C., Lunt G. G., Wonnacott S. Nicotinic modulation of [3H]dopamine release from striatal synaptosomes: pharmacological characterisation. J Neurochem. 1990 Mar;54(3):937–945. doi: 10.1111/j.1471-4159.1990.tb02341.x. [DOI] [PubMed] [Google Scholar]
- Revah F., Galzi J. L., Giraudat J., Haumont P. Y., Lederer F., Changeux J. P. The noncompetitive blocker [3H]chlorpromazine labels three amino acids of the acetylcholine receptor gamma subunit: implications for the alpha-helical organization of regions MII and for the structure of the ion channel. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4675–4679. doi: 10.1073/pnas.87.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. P., Engel G., Donatsch P., Stadler P. A. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985 Jul 11;316(6024):126–131. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Schoepfer R., Whiting P., Esch F., Blacher R., Shimasaki S., Lindstrom J. cDNA clones coding for the structural subunit of a chicken brain nicotinic acetylcholine receptor. Neuron. 1988 May;1(3):241–248. doi: 10.1016/0896-6273(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Schulz D. W., Zigmond R. E. Neuronal bungarotoxin blocks the nicotinic stimulation of endogenous dopamine release from rat striatum. Neurosci Lett. 1989 Apr 10;98(3):310–316. doi: 10.1016/0304-3940(89)90420-5. [DOI] [PubMed] [Google Scholar]
- Seeman P., Lee T., Chau-Wong M., Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976 Jun 24;261(5562):717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Sernagor E., Kuhn D., Vyklicky L., Jr, Mayer M. L. Open channel block of NMDA receptor responses evoked by tricyclic antidepressants. Neuron. 1989 Mar;2(3):1221–1227. doi: 10.1016/0896-6273(89)90306-1. [DOI] [PubMed] [Google Scholar]
- Seyler L. E., Jr, Pomerleau O. F., Fertig J. B., Hunt D., Parker K. Pituitary hormone response to cigarette smoking. Pharmacol Biochem Behav. 1986 Jan;24(1):159–162. doi: 10.1016/0091-3057(86)90062-6. [DOI] [PubMed] [Google Scholar]
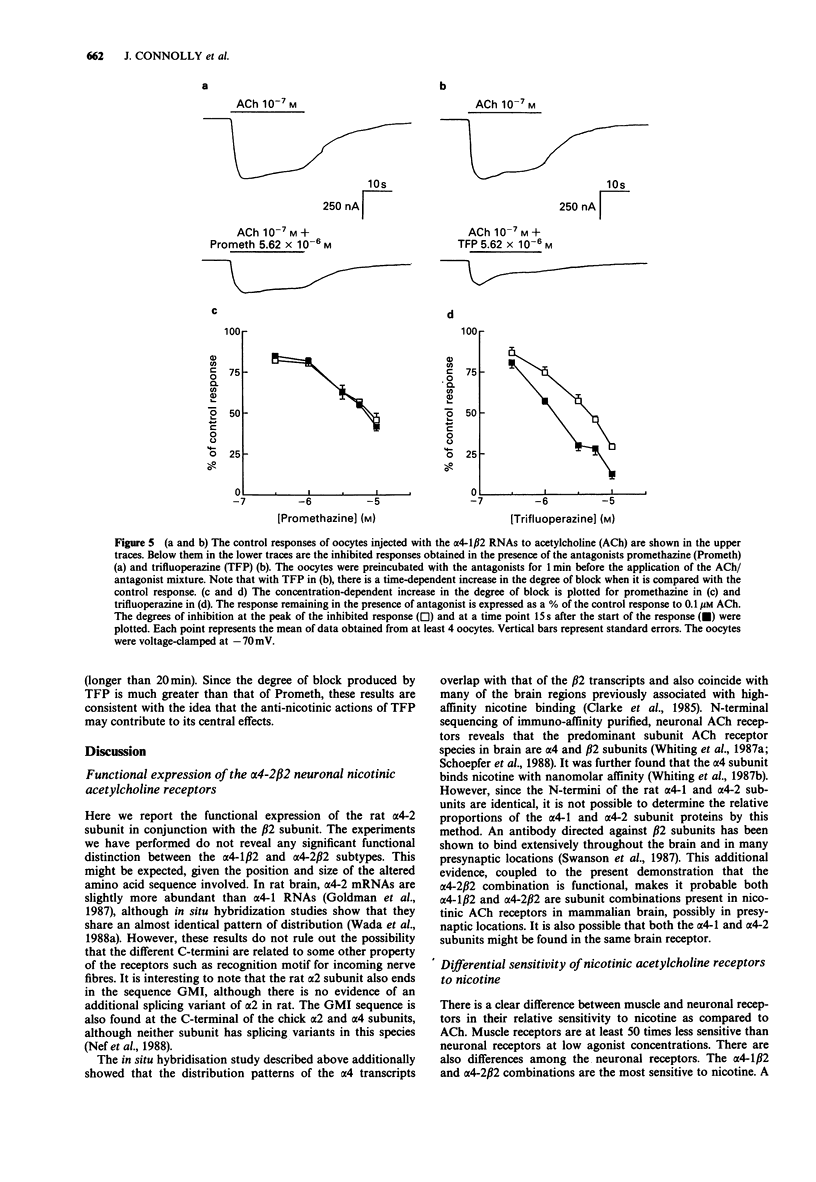
- Swanson L. W., Simmons D. M., Whiting P. J., Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987 Oct;7(10):3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y., Sakurai Y., Kohjimoto Y., Honda K., Kamiya H. O. Presynaptic modulation of the release of dopamine from striatal synaptosomes: differences in the effects of high K+ stimulation, methamphetamine and nicotinic drugs. Brain Res. 1983 Nov 21;279(1-2):330–334. doi: 10.1016/0006-8993(83)90204-4. [DOI] [PubMed] [Google Scholar]
- Vanner S., Surprenant A. Effects of 5-HT3 receptor antagonists on 5-HT and nicotinic depolarizations in guinea-pig submucosal neurones. Br J Pharmacol. 1990 Apr;99(4):840–844. doi: 10.1111/j.1476-5381.1990.tb13017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendsborg P. B., Bech P., Rafaelsen O. J. Lithium treatment and weight gain. Acta Psychiatr Scand. 1976 Feb;53(2):139–147. doi: 10.1111/j.1600-0447.1976.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Wack J. T., Rodin J. Smoking and its effects on body weight and the systems of caloric regulation. Am J Clin Nutr. 1982 Feb;35(2):366–380. doi: 10.1093/ajcn/35.2.366. [DOI] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wada K., Ballivet M., Boulter J., Connolly J., Wada E., Deneris E. S., Swanson L. W., Heinemann S., Patrick J. Functional expression of a new pharmacological subtype of brain nicotinic acetylcholine receptor. Science. 1988 Apr 15;240(4850):330–334. doi: 10.1126/science.2832952. [DOI] [PubMed] [Google Scholar]
- Wesnes K., Warburton D. M. Smoking, nicotine and human performance. Pharmacol Ther. 1983;21(2):189–208. doi: 10.1016/0163-7258(83)90072-4. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Martino A. M., Marcus K. A., Zweig R. M., Singer H. S., Price D. L., Kellar K. J. Reductions in acetylcholine and nicotine binding in several degenerative diseases. Arch Neurol. 1988 Jul;45(7):722–724. doi: 10.1001/archneur.1988.00520310028012. [DOI] [PubMed] [Google Scholar]
- Whiting P. J., Liu R., Morley B. J., Lindstrom J. M. Structurally different neuronal nicotinic acetylcholine receptor subtypes purified and characterized using monoclonal antibodies. J Neurosci. 1987 Dec;7(12):4005–4016. doi: 10.1523/JNEUROSCI.07-12-04005.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P., Esch F., Shimasaki S., Lindstrom J. Neuronal nicotinic acetylcholine receptor beta-subunit is coded for by the cDNA clone alpha 4. FEBS Lett. 1987 Jul 27;219(2):459–463. doi: 10.1016/0014-5793(87)80272-7. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Jackson M. B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988 Sep;1(7):615–621. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Yoshii K., Yu L., Mayne K. M., Davidson N., Lester H. A. Equilibrium properties of mouse-Torpedo acetylcholine receptor hybrids expressed in Xenopus oocytes. J Gen Physiol. 1987 Oct;90(4):553–573. doi: 10.1085/jgp.90.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]



