Abstract
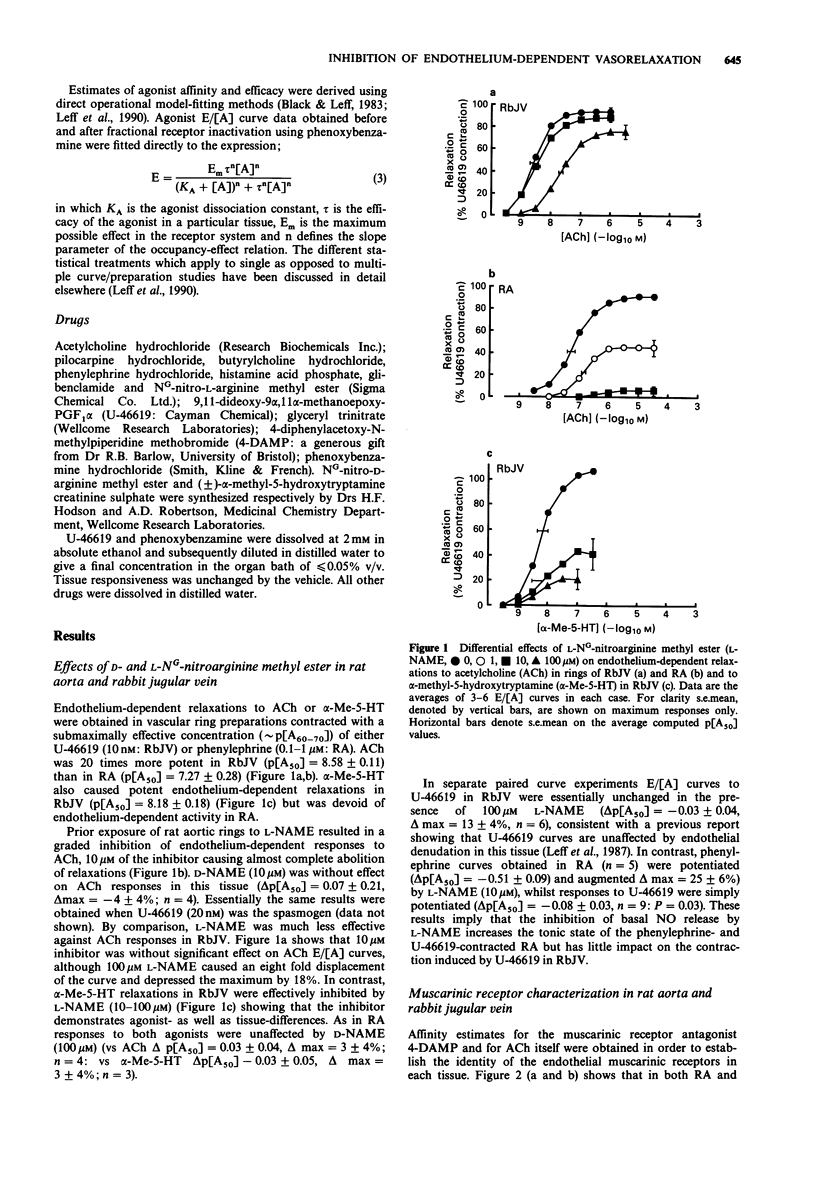
1. Isolated rings of rabbit external jugular vein (RbJV) and rat thoracic aorta (RA) were used to study the effect of the NO synthase inhibitor L-NG-nitroarginine methyl ester (L-NAME) on muscarinic and 5-hydroxytryptamine (5-HT) receptor-stimulated, endothelium-dependent vascular relaxations. 2. In RbJV relaxations produced by the endothelial 5-HT receptor agonist alpha-methyl-5-HT were potently and non-surmountably inhibited by L-NAME (10 microM), whereas acetylcholine relaxations in this tissue were unaffected by this concentration of inhibitor. By contrast, acetylcholine relaxations in RA were virtually abolished by 10 microM L-NAME. In each case an equivalent concentration of D-NAME was without effect on agonist-induced relaxations. 3. The different effect of L-NAME on acetylcholine relaxations in RbJV and RA was not due to muscarinic receptor differences. Affinity estimates for acetylcholine (pKA = 6.12 +/- 0.09; 6.09 +/- 0.08 respectively) and for 4-diphenyl-acetoxy-N-methylpiperidine methobromide (4-DAMP, pKB = 9.01 +2- 0.012; 9.24 +/- 0.16 respectively) indicated that the receptors in both tissues belong to the same M3 class. Tissue differences resulting from the release of a cyclo-oxygenase product or a glibenclamide-sensitive K(+)-channel-linked hyperpolarizing factor were also ruled out by selective inhibition of these pathways. 4. When phenoxybenzamine was used to reduce the efficacy of acetylcholine in RbJV so that it behaved as a partial agonist in this tissue, L-NAME (10 microM) now produced non-surmountable inhibition of relaxation responses. In untreated tissues the same concentration of L-NAME also profoundly inhibited responses produced by butyrylcholine and pilocarpine, both of which behave as partial agonists at the M3 receptor in RbJV. 5. A simple model was developed which describes the theoretical behaviour of receptor-stimulated synthesis and release of NO. The model predicts that competitive inhibition of NO formation results in parallel displacements of the agonist response curve in the case of high efficacy agonist, but right-shift with concomitant depression of the curve maximum in the case of low efficacy agonists. Simulations based on the model showed reasonable agreement with the experimental data. 6. It is concluded that analogues of L-arginine demonstrate tissue- and agonist-dependence in terms of their ability to inhibit receptor-mediated events involving the liberation of NO. This behaviour can reflect differences in agonist efficacy in the receptor systems being studied, a possibility that should be ruled out before apparent resistance to inhibition is taken as evidence for the involvement of heterogeneous endothelium-derived relaxing factors (EDRFs).
Full text
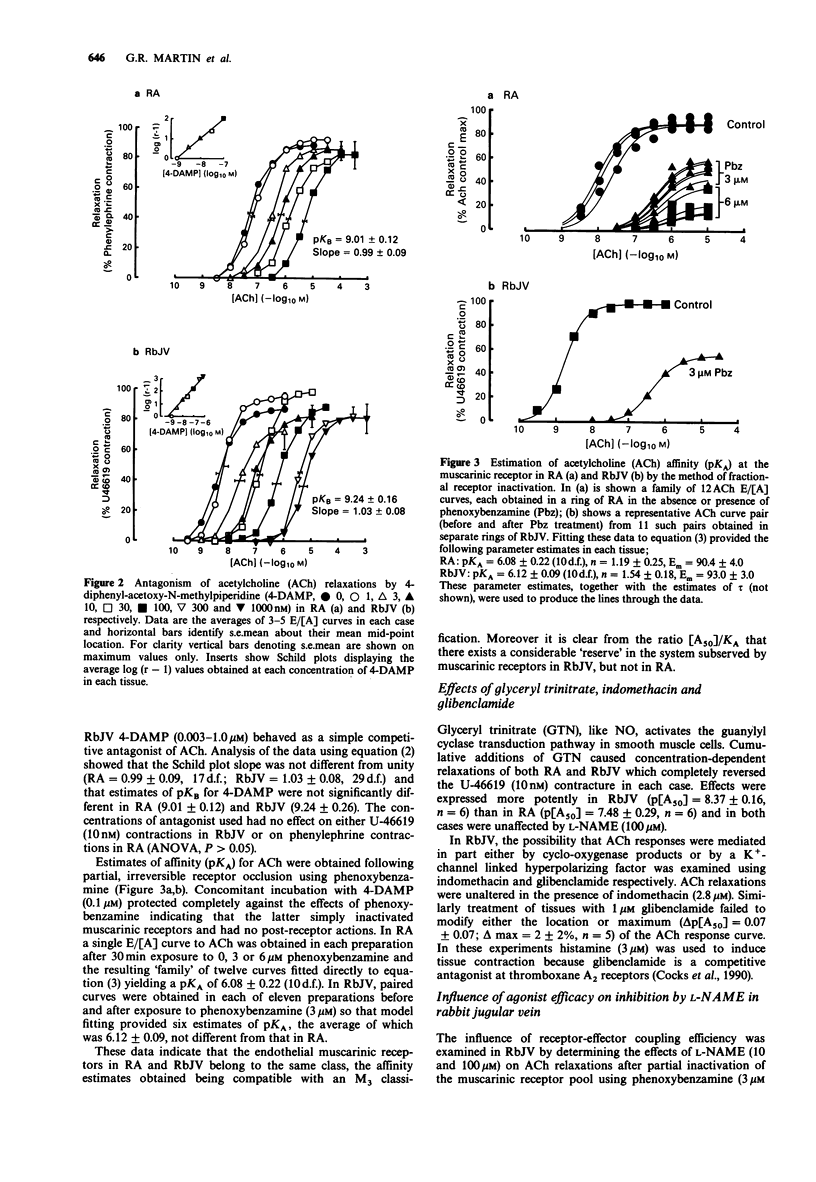
PDF

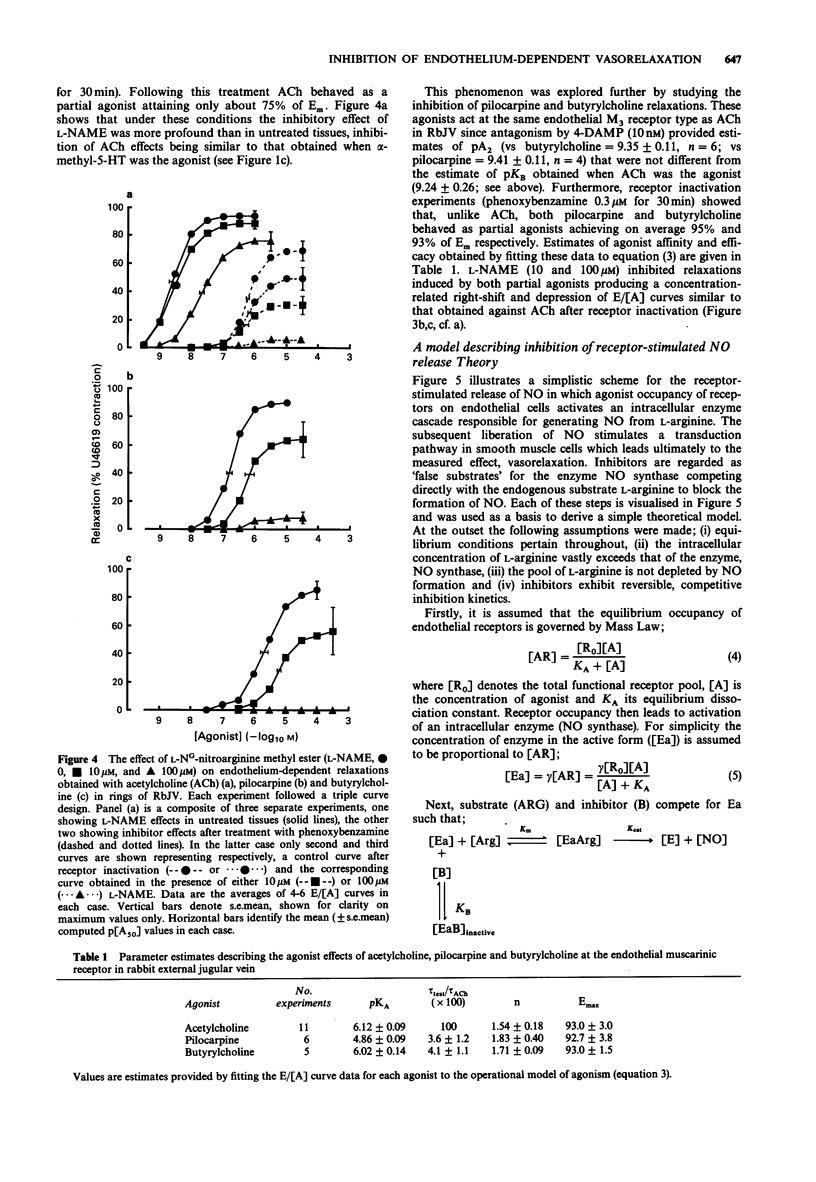



Selected References
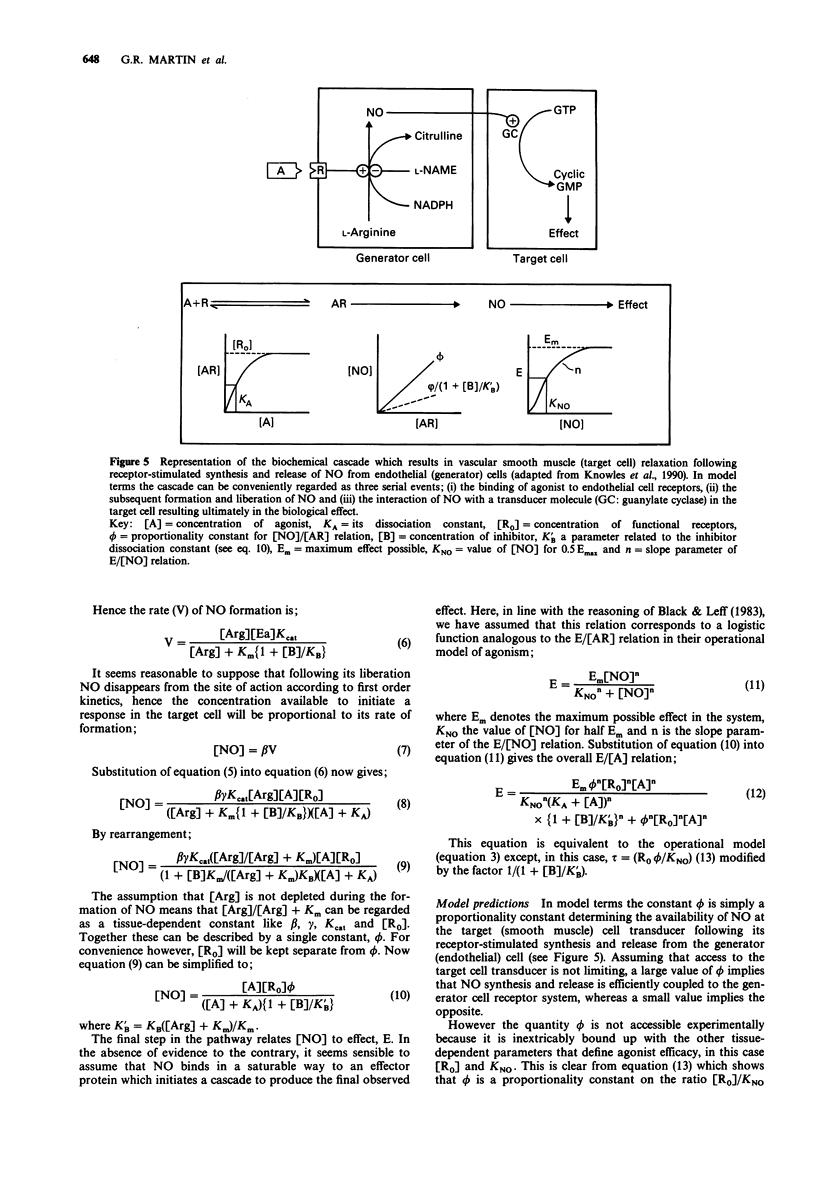
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Jenkinson D. H., Kenakin T. P. Antagonism of an indirectly acting agonist: block by propranolol and sotalol of the action of tyramine on rat heart. Eur J Pharmacol. 1980 Jul 11;65(1):1–10. doi: 10.1016/0014-2999(80)90202-2. [DOI] [PubMed] [Google Scholar]
- Black J. W., Jenkinson D. H., Welch L. Analysis of the interaction between histamine H2-receptor antagonists and pentagastrin [proceedings]. Agents Actions. 1978 Jun;8(4):383–384. doi: 10.1007/BF01968624. [DOI] [PubMed] [Google Scholar]
- Black J. W., Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983 Dec 22;220(1219):141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P. Pharmacological analysis of the pentagastrin-tiotidine interaction in the mouse isolated stomach. Br J Pharmacol. 1985 Nov;86(3):589–599. doi: 10.1111/j.1476-5381.1985.tb08935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Leff P., Shankley N. P., Wood J. An operational model of pharmacological agonism: the effect of E/[A] curve shape on agonist dissociation constant estimation. Br J Pharmacol. 1985 Feb;84(2):561–571. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C., Hendrickson H., Lorenz R. R., Vanhoutte P. M. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res. 1989 Jun;64(6):1070–1078. doi: 10.1161/01.res.64.6.1070. [DOI] [PubMed] [Google Scholar]
- Choo L. K., Malta E., Mitchelson F. The affinity of some selective muscarinic receptor antagonists for the muscarinic receptor mediating endothelial-dependent relaxation of the rabbit and rat thoracic aorta. J Pharm Pharmacol. 1986 Nov;38(11):843–845. doi: 10.1111/j.2042-7158.1986.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Cocks T. M., King S. J., Angus J. A. Glibenclamide is a competitive antagonist of the thromboxane A2 receptor in dog coronary artery in vitro. Br J Pharmacol. 1990 Jun;100(2):375–378. doi: 10.1111/j.1476-5381.1990.tb15812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East S. J., Garthwaite J. Nanomolar N(G)-nitroarginine inhibits NMDA-induced cyclic GMP formation in rat cerebellum. Eur J Pharmacol. 1990 Aug 10;184(2-3):311–313. doi: 10.1016/0014-2999(90)90623-e. [DOI] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Muscarinic receptor subtypes: a critique of the current classification and a proposal for a working nomenclature. J Auton Pharmacol. 1986 Dec;6(4):323–346. doi: 10.1111/j.1474-8673.1986.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Fedan J. S., Besse J. C., Carpenter F. G., Teague R. S. Motor innervation of the smooth muscle of the rat seminal vesicle. J Pharmacol Exp Ther. 1977 May;201(2):285–297. [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Palmer R. M., Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989 Oct 17;172(4-5):413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Hoeffner U., Feletou M., Flavahan N. A., Vanhoutte P. M. Canine arteries release two different endothelium-derived relaxing factors. Am J Physiol. 1989 Jul;257(1 Pt 2):H330–H333. doi: 10.1152/ajpheart.1989.257.1.H330. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Kinetic characteristics of nitric oxide synthase from rat brain. Biochem J. 1990 Jul 1;269(1):207–210. doi: 10.1042/bj2690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff P., Martin G. R., Morse J. M. Differential classification of vascular smooth muscle and endothelial cell 5-HT receptors by use of tryptamine analogues. Br J Pharmacol. 1987 Jun;91(2):321–331. doi: 10.1111/j.1476-5381.1987.tb10287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff P., Prentice D. J., Giles H., Martin G. R., Wood J. Estimation of agonist affinity and efficacy by direct, operational model-fitting. J Pharmacol Methods. 1990 May;23(3):225–237. doi: 10.1016/0160-5402(90)90066-t. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Leff P., Cambridge D., Barrett V. J. Comparative analysis of two types of 5-hydroxytryptamine receptor mediating vasorelaxation: differential classification using tryptamines. Naunyn Schmiedebergs Arch Pharmacol. 1987 Oct;336(4):365–373. doi: 10.1007/BF00164867. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Feelisch M., Palmer R. M., Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991 Jan;102(1):234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palacios M., Knowles R. G., Palmer R. M., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989 Dec 15;165(2):802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Characterization of the L-arginine:nitric oxide pathway in human platelets. Br J Pharmacol. 1990 Oct;101(2):325–328. doi: 10.1111/j.1476-5381.1990.tb12709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Trist D. G., Leff P. Quantification of H2-agonism by clonidine and dimaprit in an adenylate cyclase assay. Agents Actions. 1985 Apr;16(3-4):222–226. doi: 10.1007/BF01983145. [DOI] [PubMed] [Google Scholar]



