Abstract
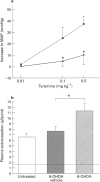
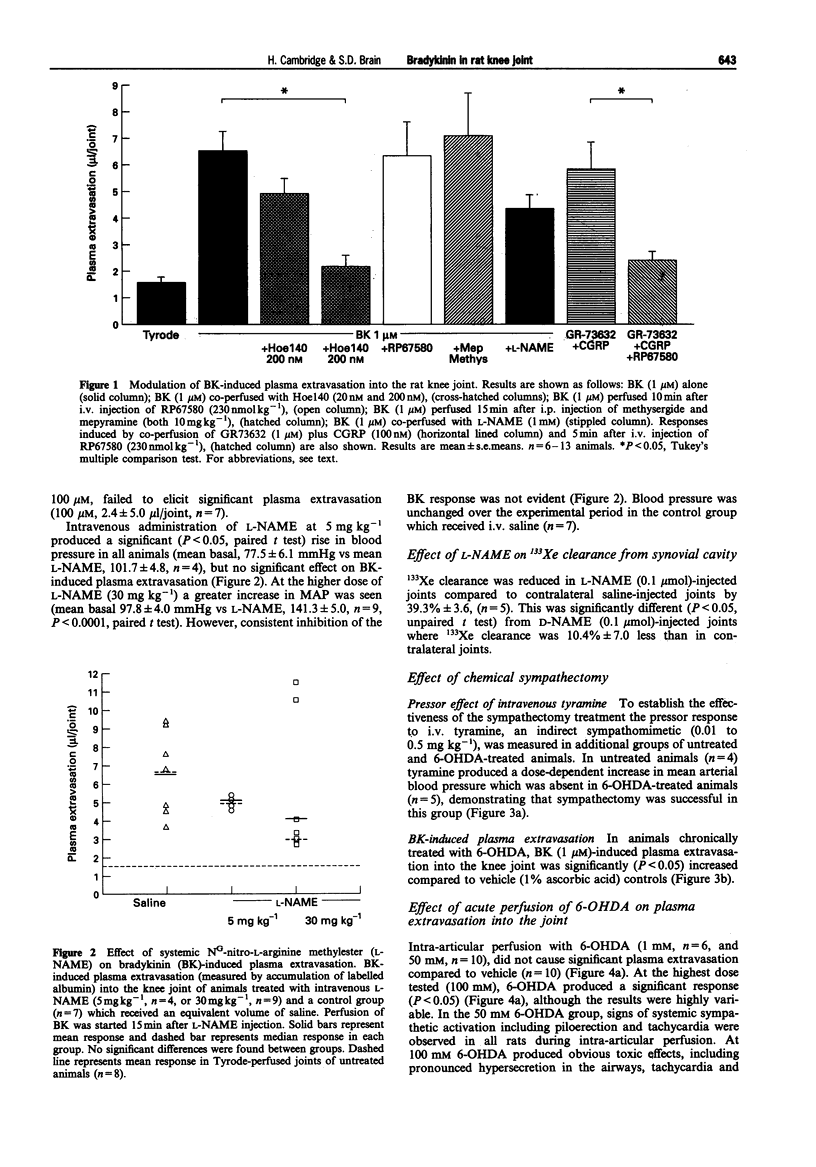
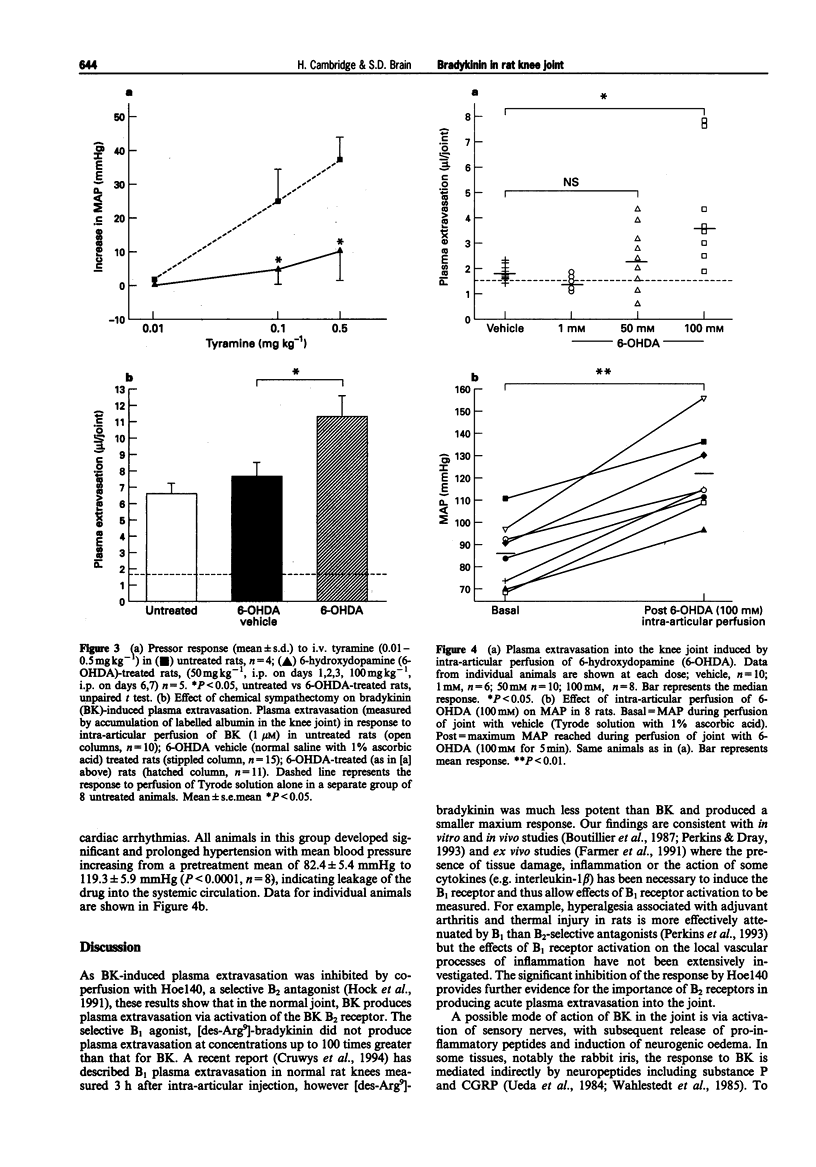
1. We have investigated the mechanism of bradykinin (BK)-induced plasma extravasation into the knee joint of the anaesthetized rat. Accumulation of [125I]-human serum albumin within the synovial cavity was used as a marker of increased vascular permeability. 2. Perfusion with BK (1 microM) produced significant plasma extravasation into the knee which was inhibited by co-perfusion of the selective bradykinin B2 receptor antagonist D-Arg-[Hyp3,Thi5,D-Tic7,Oic8]-bradykinin (Hoe 140, 200 nM). 3. The bradykinin B1 receptor agonist, [des-Arg9]-BK (up to 100 mM), did not induce plasma extravasation into the knee joint, over this time period. 4. Chemical sympathectomy by chronically administered 6-hydroxydopamine (6-OHDA) did not inhibit bradykinin-induced plasma extravasation. Acute intra-articular perfusion with 6-OHDA (to stimulate transmitter release from sympathetic nerve terminals) at concentrations up to 50 mM did not induce significant plasma extravasation. Intra-articular perfusion of 100 mM 6-OHDA induced significant plasma extravasation but produced severe systemic toxicity. 5. The selective neurokinin1 (NK1) receptor antagonist, RP67580 (230 nmol kg-1), or receptor antagonists for the mast cell products histamine and 5-hydroxytryptamine did not significantly inhibit BK-induced plasma extravasation. 6. Co-perfusion of the NO synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME) (1 mM) did not significantly inhibit the response to BK. 133Xe clearance from L-NAME (1 mM)-injected joints was significantly (P < 0.05) reduced compared to D-NAME injected joints, suggesting a reduction in blood flow as a result of decreased basal NO production.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaujouan J. C., Heuillet E., Petitet F., Saffroy M., Torrens Y., Glowinski J. Higher potency of RP 67580, in the mouse and the rat compared with other nonpeptide and peptide tachykinin NK1 antagonists. Br J Pharmacol. 1993 Mar;108(3):793–800. doi: 10.1111/j.1476-5381.1993.tb12880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes L., Coderre T. J., Green P. G., Basbaum A. I., Levine J. D. Neutrophils contribute to sympathetic nerve terminal-dependent plasma extravasation in the knee joint of the rat. Neuroscience. 1991;43(2-3):679–685. doi: 10.1016/0306-4522(91)90326-j. [DOI] [PubMed] [Google Scholar]
- Bouthillier J., Deblois D., Marceau F. Studies on the induction of pharmacological responses to des-Arg9-bradykinin in vitro and in vivo. Br J Pharmacol. 1987 Oct;92(2):257–264. doi: 10.1111/j.1476-5381.1987.tb11319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol. 1985 Dec;86(4):855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bültmann R., Starke K. Evans blue blocks P2X-purinoceptors in rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1993 Dec;348(6):684–687. doi: 10.1007/BF00167248. [DOI] [PubMed] [Google Scholar]
- Cambridge H., Brain S. D. Calcitonin gene-related peptide increases blood flow and potentiates plasma protein extravasation in the rat knee joint. Br J Pharmacol. 1992 Jul;106(3):746–750. doi: 10.1111/j.1476-5381.1992.tb14404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre T. J., Basbaum A. I., Levine J. D. Neural control of vascular permeability: interactions between primary afferents, mast cells, and sympathetic efferents. J Neurophysiol. 1989 Jul;62(1):48–58. doi: 10.1152/jn.1989.62.1.48. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Chan A. K., Helms C., Basbaum A. I., Levine J. D. Increasing sympathetic nerve terminal-dependent plasma extravasation correlates with decreased arthritic joint injury in rats. Neuroscience. 1991;40(1):185–189. doi: 10.1016/0306-4522(91)90184-p. [DOI] [PubMed] [Google Scholar]
- Cruwys S. C., Garrett N. E., Perkins M. N., Blake D. R., Kidd B. L. The role of bradykinin B1 receptors in the maintenance of intra-articular plasma extravasation in chronic antigen-induced arthritis. Br J Pharmacol. 1994 Nov;113(3):940–944. doi: 10.1111/j.1476-5381.1994.tb17083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruwys S. C., Kidd B. L., Mapp P. I., Walsh D. A., Blake D. R. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharmacol. 1992 Sep;107(1):116–119. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnerer J., Amann R., Lembeck F. Neurogenic and non-neurogenic inflammation in the rat paw following chemical sympathectomy. Neuroscience. 1991;45(3):761–765. doi: 10.1016/0306-4522(91)90288-y. [DOI] [PubMed] [Google Scholar]
- Dray A., Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993 Mar;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., McMillan B. A., Meeker S. N., Burch R. M. Induction of vascular smooth muscle bradykinin B1 receptors in vivo during antigen arthritis. Agents Actions. 1991 Sep;34(1-2):191–193. doi: 10.1007/BF01993275. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1990 Nov;101(3):625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garret C., Carruette A., Fardin V., Moussaoui S., Peyronel J. F., Blanchard J. C., Laduron P. M. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. G., Basbaum A. I., Helms C., Levine J. D. Purinergic regulation of bradykinin-induced plasma extravasation and adjuvant-induced arthritis in the rat. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4162–4165. doi: 10.1073/pnas.88.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. G., Luo J., Heller P. H., Levine J. D. Further substantiation of a significant role for the sympathetic nervous system in inflammation. Neuroscience. 1993 Aug;55(4):1037–1043. doi: 10.1016/0306-4522(93)90317-9. [DOI] [PubMed] [Google Scholar]
- Green P. G., Luo J., Heller P., Levine J. D. Modulation of bradykinin-induced plasma extravasation in the rat knee joint by sympathetic co-transmitters. Neuroscience. 1993 Jan;52(2):451–458. doi: 10.1016/0306-4522(93)90171-b. [DOI] [PubMed] [Google Scholar]
- Hall J. M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992 Nov;56(2):131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hock F. J., Wirth K., Albus U., Linz W., Gerhards H. J., Wiemer G., Henke S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br J Pharmacol. 1991 Mar;102(3):769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. R., Williams T. J., Brain S. D. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990 Dec 4;191(3):481–484. doi: 10.1016/0014-2999(90)94184-y. [DOI] [PubMed] [Google Scholar]
- Jones S., Brown D. A., Milligan G., Willer E., Buckley N. J., Caulfield M. P. Bradykinin excites rat sympathetic neurons by inhibition of M current through a mechanism involving B2 receptors and G alpha q/11. Neuron. 1995 Feb;14(2):399–405. doi: 10.1016/0896-6273(95)90295-3. [DOI] [PubMed] [Google Scholar]
- Khalil Z., Helme R. D. Sympathetic neurons modulate plasma extravasation in the rat through a non-adrenergic mechanism. Clin Exp Neurol. 1989;26:45–50. [PubMed] [Google Scholar]
- Khalil Z., Helme R. D. The quantitative contribution of nitric oxide and sensory nerves to bradykinin-induced inflammation in rat skin microvasculature. Brain Res. 1992 Aug 28;589(1):102–108. doi: 10.1016/0006-8993(92)91167-d. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M., Kress M., Reeh P. W. The nociceptor sensitization by bradykinin does not depend on sympathetic neurons. Neuroscience. 1992;46(2):465–473. doi: 10.1016/0306-4522(92)90066-b. [DOI] [PubMed] [Google Scholar]
- Mapp P. I., Kidd B. L., Gibson S. J., Terry J. M., Revell P. A., Ibrahim N. B., Blake D. R., Polak J. M. Substance P-, calcitonin gene-related peptide- and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience. 1990;37(1):143–153. doi: 10.1016/0306-4522(90)90199-e. [DOI] [PubMed] [Google Scholar]
- Najafipour H., Ferrell W. R. Sympathetic innervation and beta-adrenoceptor profile of blood vessels in the posterior region of the rabbit knee joint. Exp Physiol. 1993 Sep;78(5):625–637. doi: 10.1113/expphysiol.1993.sp003711. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Campbell E., Dray A. Antinociceptive activity of the bradykinin B1 and B2 receptor antagonists, des-Arg9, [Leu8]-BK and HOE 140, in two models of persistent hyperalgesia in the rat. Pain. 1993 May;53(2):191–197. doi: 10.1016/0304-3959(93)90080-9. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepheard S. L., Williamson D. J., Hill R. G., Hargreaves R. J. The non-peptide neurokinin1 receptor antagonist, RP 67580, blocks neurogenic plasma extravasation in the dura mater of rats. Br J Pharmacol. 1993 Jan;108(1):11–12. doi: 10.1111/j.1476-5381.1993.tb13432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulakvelidze I., Baluk P., McDonald D. M. Plasma extravasation induced in rat trachea by 6-OHDA is mediated by sensory nerves, not by sympathetic nerves. J Appl Physiol (1985) 1994 Feb;76(2):701–707. doi: 10.1152/jappl.1994.76.2.701. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Tranzer J. P. Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-Hydroxydopamine. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;261(3):271–288. doi: 10.1007/BF00536990. [DOI] [PubMed] [Google Scholar]
- Toda N., Kitamura Y., Okamura T. Neural mechanism of hypertension by nitric oxide synthase inhibitor in dogs. Hypertension. 1993 Jan;21(1):3–8. doi: 10.1161/01.hyp.21.1.3. [DOI] [PubMed] [Google Scholar]
- Ueda N., Muramatsu I., Fujiwara M. Capsaicin and bradykinin-induced substance P-ergic responses in the iris sphincter muscle of the rabbit. J Pharmacol Exp Ther. 1984 Aug;230(2):469–473. [PubMed] [Google Scholar]
- Wahlestedt C., Bynke G., Håkanson R. Pupillary constriction by bradykinin and capsaicin: mode of action. Eur J Pharmacol. 1984 Nov 27;106(3):577–583. doi: 10.1016/0014-2999(84)90061-x. [DOI] [PubMed] [Google Scholar]
- Weiss C., Sela D., Atlas D. Divalent cations effectively replace Ca2+ and support bradykinin induced noradrenaline release. Neurosci Lett. 1990 Nov 13;119(2):241–244. doi: 10.1016/0304-3940(90)90843-x. [DOI] [PubMed] [Google Scholar]