Abstract
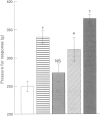
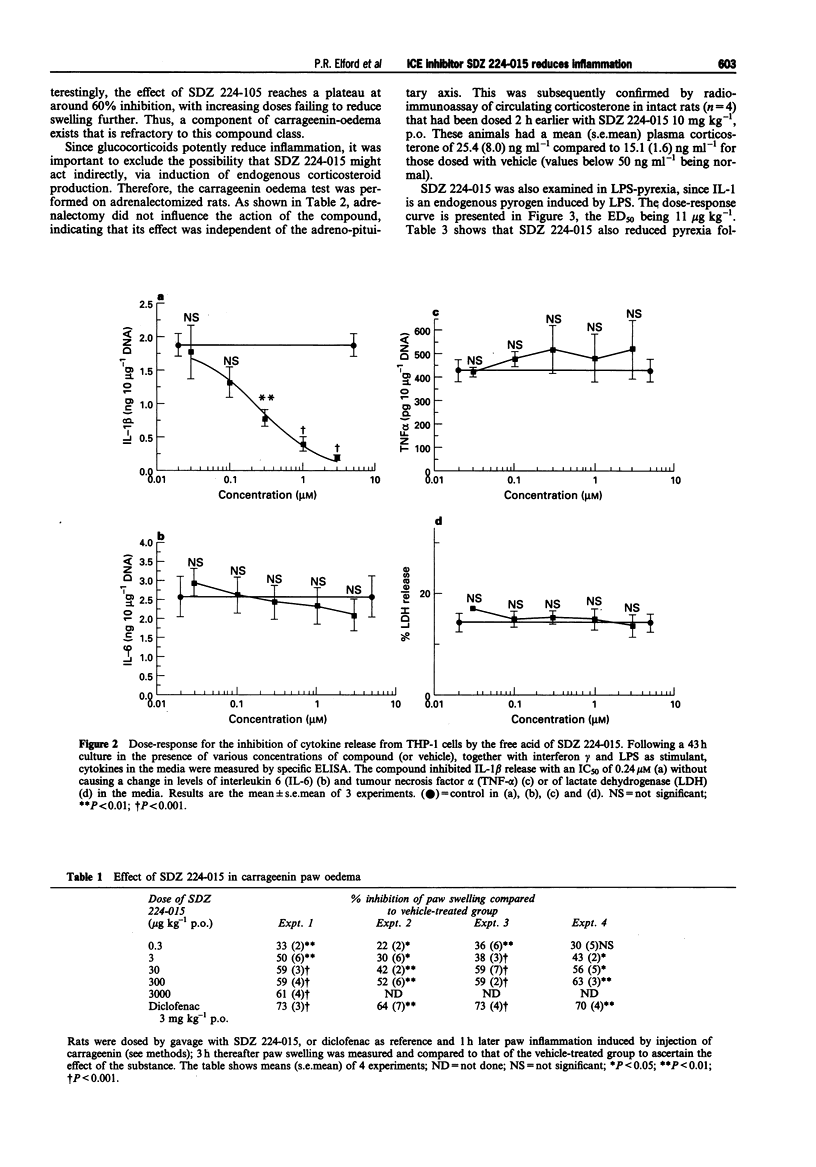
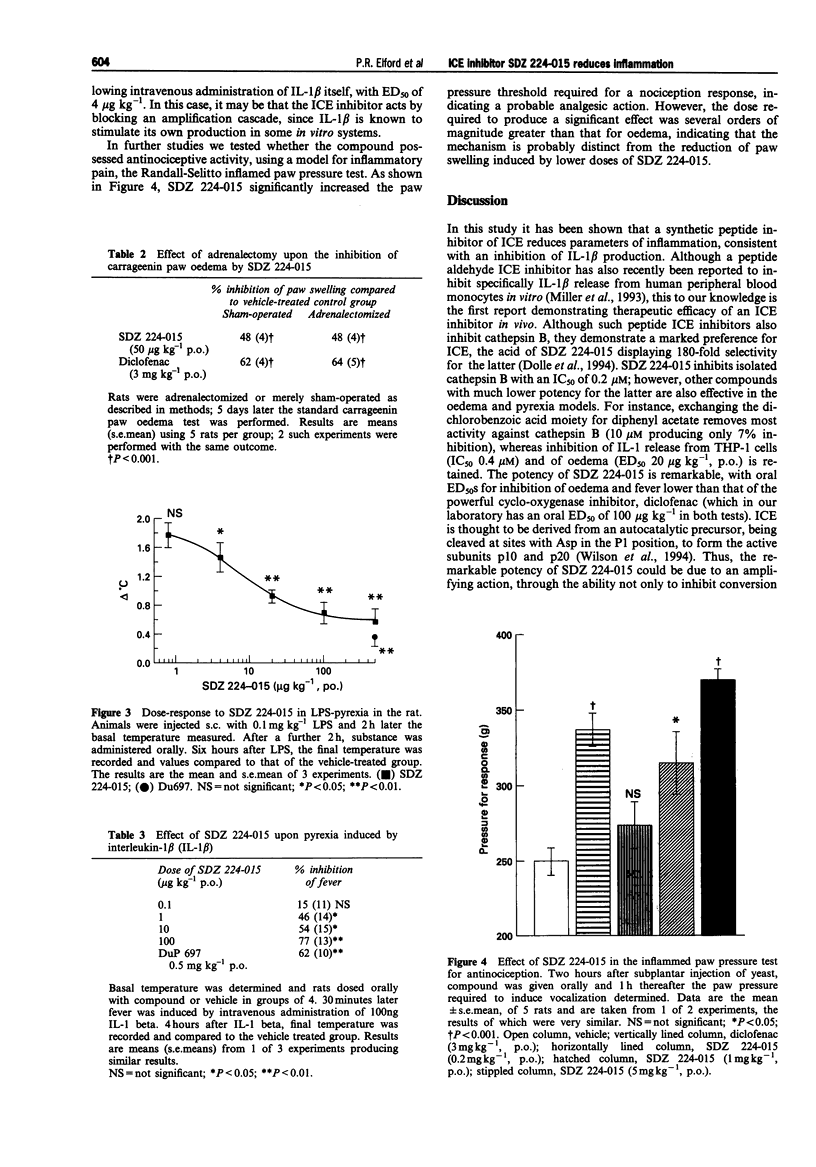
1. The aim of this study was to determine whether a synthetic inhibitor of the interleukin-1 beta converting enzyme (ICE) displays oral activity in models of inflammation. 2. To this end, the ICE inhibitor, SDZ 224-015, was examined in rat paw oedema, pyrexia and nociception tests. 3. SDZ 224-015 (0.3-300 micrograms kg-1) potently reduced carrageenin-induced paw oedema, with an oral ED50 of approximately 25 micrograms kg-1. This effect was independent of endogenous glucocorticoid, as shown by retention of activity upon adrenalectomy. 4. Pyrexia induced by lipopolysaccharide (0.1 mg kg-1 s.c.) or by interleukin-1 beta (100 ng i.v.) was also reduced, over a similar dose-range to oedema (oral ED50s 11 micrograms kg-1 and 4 micrograms kg-1 respectively). 5. SDZ 224-015 (0.2-5 mg kg-1, p.o.) displayed analgesic activity in the Randall-Selitto yeast-inflamed paw pressure test, significant at a dose of 1 mg kg-1, p.o. 6. Thus, SDZ 224-015 has potent oral activity in several acute models for inflammation, suggesting that ICE inhibitors may constitute a novel type of anti-inflammatory agent.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayala J. M., Yamin T. T., Egger L. A., Chin J., Kostura M. J., Miller D. K. IL-1 beta-converting enzyme is present in monocytic cells as an inactive 45-kDa precursor. J Immunol. 1994 Sep 15;153(6):2592–2599. [PubMed] [Google Scholar]
- Bailly S., Fay M., Ferrua B., Gougerot-Pocidalo M. A. Comparative production of IL-1 beta and IL-1 alpha by LPS-stimulated human monocytes: ELISAs measurement revisited. Cytokine. 1994 Mar;6(2):111–115. doi: 10.1016/1043-4666(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Bochner B. S., Charlesworth E. N., Lichtenstein L. M., Derse C. P., Gillis S., Dinarello C. A., Schleimer R. P. Interleukin-1 is released at sites of human cutaneous allergic reactions. J Allergy Clin Immunol. 1990 Dec;86(6 Pt 1):830–839. doi: 10.1016/s0091-6749(05)80143-5. [DOI] [PubMed] [Google Scholar]
- Coceani F., Lees J., Redford J., Bishai I. Interleukin-1 receptor antagonist: effectiveness against interleukin-1 fever. Can J Physiol Pharmacol. 1992 Dec;70(12):1590–1596. doi: 10.1139/y92-228. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Dolle R. E., Hoyer D., Prasad C. V., Schmidt S. J., Helaszek C. T., Miller R. E., Ator M. A. P1 aspartate-based peptide alpha-((2,6-dichlorobenzoyl)oxy)methyl ketones as potent time-dependent inhibitors of interleukin-1 beta-converting enzyme. J Med Chem. 1994 Mar 4;37(5):563–564. doi: 10.1021/jm00031a003. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Lorenzetti B. B., Bristow A. F., Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988 Aug 25;334(6184):698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- Gagliardini V., Fernandez P. A., Lee R. K., Drexler H. C., Rotello R. J., Fishman M. C., Yuan J. Prevention of vertebrate neuronal death by the crmA gene. Science. 1994 Feb 11;263(5148):826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- Gans K. R., Galbraith W., Roman R. J., Haber S. B., Kerr J. S., Schmidt W. K., Smith C., Hewes W. E., Ackerman N. R. Anti-inflammatory and safety profile of DuP 697, a novel orally effective prostaglandin synthesis inhibitor. J Pharmacol Exp Ther. 1990 Jul;254(1):180–187. [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R. Comparison of the in vivo inflammatory activities after intra-articular injection of natural and recombinant IL-1 alpha and IL-1 beta in the rabbit. Biochem Pharmacol. 1988 Nov 1;37(21):4171–4176. doi: 10.1016/0006-2952(88)90112-8. [DOI] [PubMed] [Google Scholar]
- Jacobs C. A., Baker P. E., Roux E. R., Picha K. S., Toivola B., Waugh S., Kennedy M. K. Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha and suppressed by soluble IL-1 receptor. J Immunol. 1991 May 1;146(9):2983–2989. [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Kimble R. B., Vannice J. L., Bloedow D. C., Thompson R. C., Hopfer W., Kung V. T., Brownfield C., Pacifici R. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J Clin Invest. 1994 May;93(5):1959–1967. doi: 10.1172/JCI117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. Subversion of the immune system by pathogens. Cell. 1994 Jan 28;76(2):323–332. doi: 10.1016/0092-8674(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Meyers K. P., Czachowski C. L., Coffey J. W. Effect of treatment with interleukin-1 receptor antagonist on the development of carrageenan-induced pleurisy in the rat. Inflammation. 1993 Apr;17(2):121–134. doi: 10.1007/BF00916099. [DOI] [PubMed] [Google Scholar]
- Miller D. K., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Molineaux S. M., Thornberry N. A. The IL-1 beta converting enzyme as a therapeutic target. Ann N Y Acad Sci. 1993 Nov 30;696:133–148. doi: 10.1111/j.1749-6632.1993.tb17148.x. [DOI] [PubMed] [Google Scholar]
- Perretti M., Ahluwalia A., Flower R. J., Manzini S. Endogenous tachykinins play a role in IL-1-induced neutrophil accumulation: involvement of NK-1 receptors. Immunology. 1993 Sep;80(1):73–77. [PMC free article] [PubMed] [Google Scholar]
- RANDALL L. O., SELITTO J. J. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957 Sep 1;111(4):409–419. [PubMed] [Google Scholar]
- Relton J. K., Rothwell N. J. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992 Aug;29(2):243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Sakai K., Hattori T., Matsuoka M., Asou N., Yamamoto S., Sagawa K., Takatsuki K. Autocrine stimulation of interleukin 1 beta in acute myelogenous leukemia cells. J Exp Med. 1987 Nov 1;166(5):1597–1602. doi: 10.1084/jem.166.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J., Bollinger P., Payne T. Inhibition of interleukin-1 release by IX 207-887. Agents Actions. 1990 Jun;30(3-4):350–362. doi: 10.1007/BF01966299. [DOI] [PubMed] [Google Scholar]
- Sim T. C., Grant J. A., Hilsmeier K. A., Fukuda Y., Alam R. Proinflammatory cytokines in nasal secretions of allergic subjects after antigen challenge. Am J Respir Crit Care Med. 1994 Feb;149(2 Pt 1):339–344. doi: 10.1164/ajrccm.149.2.8306027. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Dripps D. J., Eisenberg S. P. Interleukin-1 receptor antagonist (IL-1ra) as a probe and as a treatment for IL-1 mediated disease. Int J Immunopharmacol. 1992 Apr;14(3):475–480. doi: 10.1016/0192-0561(92)90178-n. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Walker N. P., Talanian R. V., Brady K. D., Dang L. C., Bump N. J., Ferenz C. R., Franklin S., Ghayur T., Hackett M. C., Hammill L. D. Crystal structure of the cysteine protease interleukin-1 beta-converting enzyme: a (p20/p10)2 homodimer. Cell. 1994 Jul 29;78(2):343–352. doi: 10.1016/0092-8674(94)90303-4. [DOI] [PubMed] [Google Scholar]
- Wankowicz Z., Megyeri P., Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988 Apr;43(4):349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]
- Wilson K. P., Black J. A., Thomson J. A., Kim E. E., Griffith J. P., Navia M. A., Murcko M. A., Chambers S. P., Aldape R. A., Raybuck S. A. Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994 Jul 28;370(6487):270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- Young P. R., Hazuda D. J., Simon P. L. Human interleukin 1 beta is not secreted from hamster fibroblasts when expressed constitutively from a transfected cDNA. J Cell Biol. 1988 Aug;107(2):447–456. doi: 10.1083/jcb.107.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg W. B., Joosten L. A., Helsen M., van de Loo F. A. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin Exp Immunol. 1994 Feb;95(2):237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]