Abstract
1. We have previously shown that the activation of either protein kinase A (PKA) or protein kinase C (PKC) enhanced the responses of muscle membrane to acetylcholine (ACh) by increasing the mean open time of embryonic-type ACh channels in Xenopus cultured myocytes. In the present study, we further investigated the interaction between these two kinases in the modulation of ACh channels by using the receptor ligands, adenosine diphosphate (ADP) and calcitonin gene-related peptide (CGRP) which selectively activate PKC and PKA, respectively. 2. ADP concentration-dependently increased the mean open time of embryonic-type ACh channels and 0.3 mM ADP is sufficient to achieve the maximal potentiating effect. alpha, beta-Methylene ATP and PMA (phorbol 12-myristate 13-acetate) but not adenosine, AMP, dibutyryl cyclic GMP have similar potentiating action. 3. Suramin (0.3 mM) pretreatment abolished the potentiating effect of ADP but left that of PMA unchanged. 4. CGRP increased the mean open time of embryonic-type ACh channels in a concentration-dependent manner and 1 microM CGRP produced the maximal effect. 5. The maximal effects of both ADP (0.3 mM) and CGRP (1 microM) in the prolongation of mean open time of ACh channels were additive. 6. These results suggest that the modulation of embryonic-type ACh channels by the endogenously released ligands via the activation of PKA and PKC is additive and possibly different sites of ACh channels may be involved in the potentiation effect of either PKC or PKA.
Full text
PDF
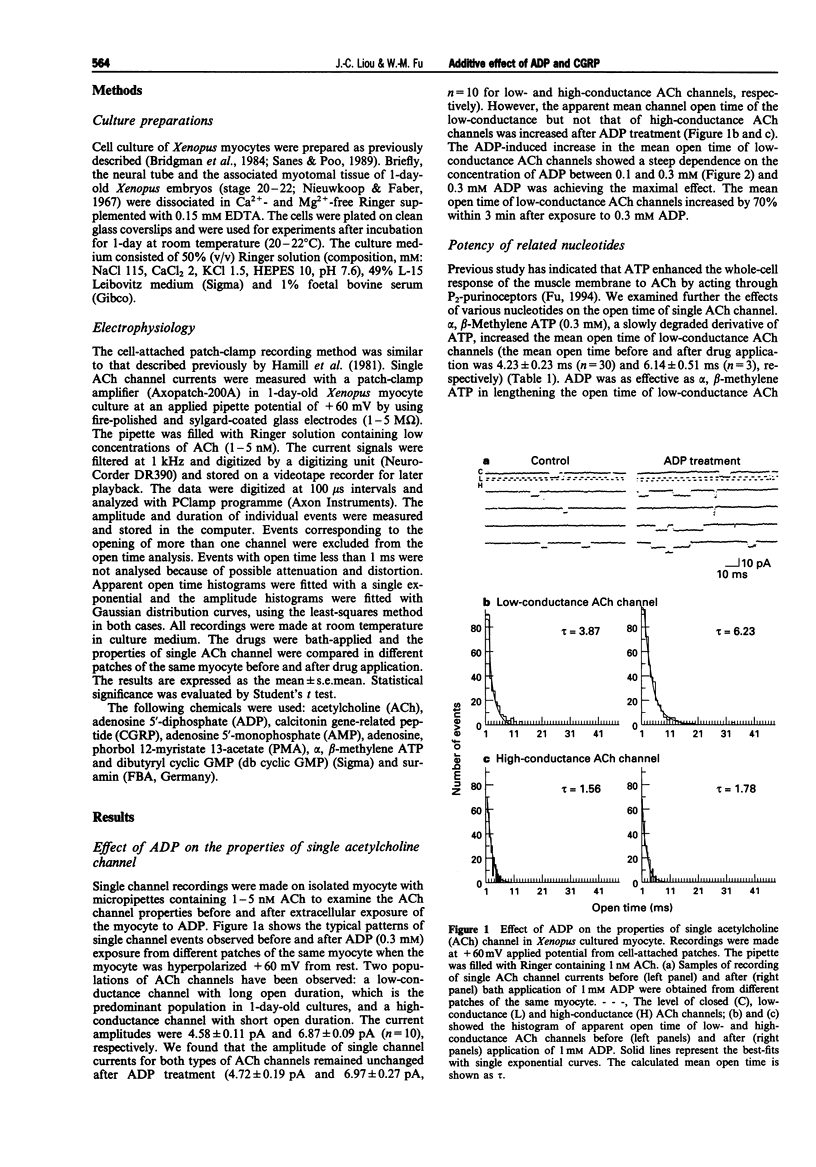
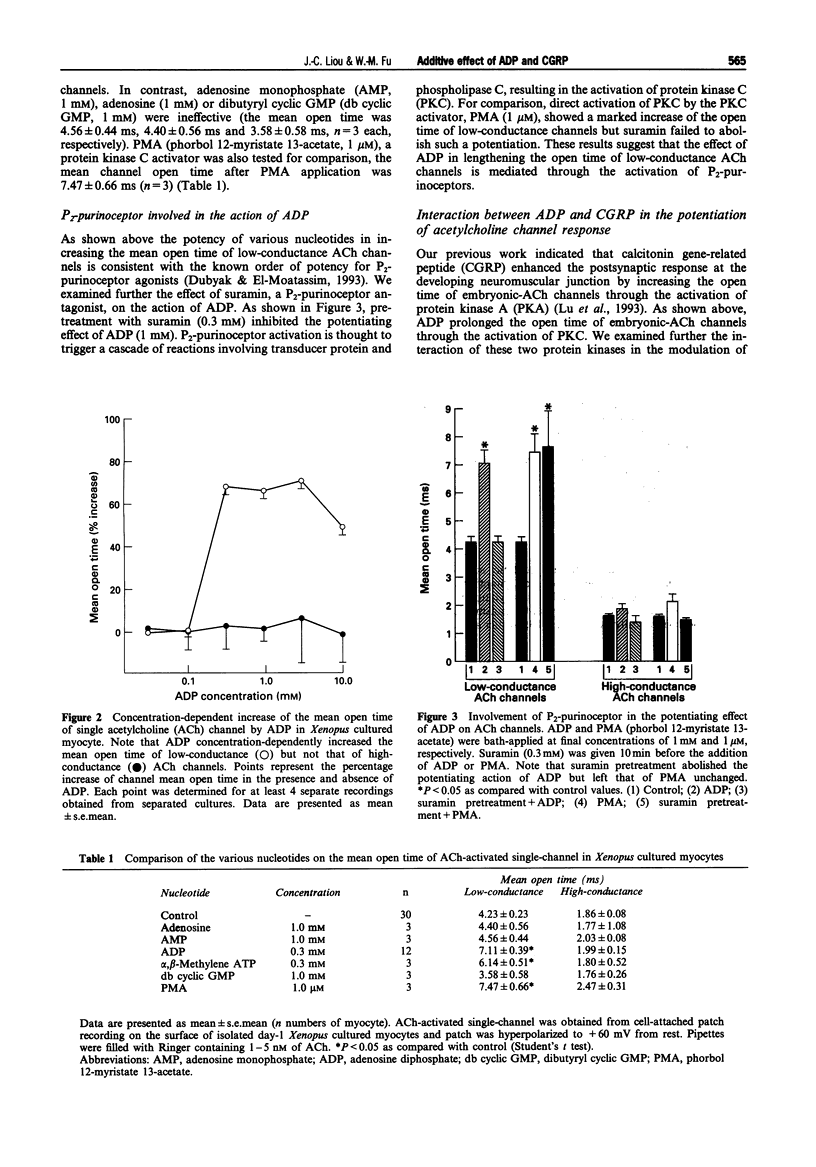


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Deshpande S. S., Aracava Y., Alkondon M., Daly J. W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. A study with forskolin and its analogs. FEBS Lett. 1986 Apr 7;199(1):113–120. doi: 10.1016/0014-5793(86)81235-2. [DOI] [PubMed] [Google Scholar]
- Brehm P., Kidokoro Y., Moody-Corbett F. Acetylcholine receptor channel properties during development of Xenopus muscle cells in culture. J Physiol. 1984 Dec;357:203–217. doi: 10.1113/jphysiol.1984.sp015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman P. C., Nakajima S., Greenberg A. S., Nakajima Y. Freeze-fracture and electrophysiological studies of newly developed acetylcholine receptors in Xenopus embryonic muscle cells. J Cell Biol. 1984 Jun;98(6):2160–2173. doi: 10.1083/jcb.98.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Dowdall M. J., Boyne A. F., Whittaker V. P. Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J. 1974 Apr;140(1):1–12. doi: 10.1042/bj1400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M., Zani B. M. Agents that activate protein kinase C reduce acetylcholine sensitivity in cultured myotubes. J Cell Biol. 1985 Apr;100(4):1339–1342. doi: 10.1083/jcb.100.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W. M., Lin J. L. Activation of protein kinase C potentiates postsynaptic acetylcholine response at developing neuromuscular synapses. Br J Pharmacol. 1993 Oct;110(2):707–712. doi: 10.1111/j.1476-5381.1993.tb13869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W. M., Lin J. L. Developmental change in the modulation of acetylcholine receptor channel by protein kinase C activation in Xenopus embryonic muscle cells. Neurosci Lett. 1993 Dec 24;164(1-2):97–100. doi: 10.1016/0304-3940(93)90866-j. [DOI] [PubMed] [Google Scholar]
- Fu W. M., Poo M. M. ATP potentiates spontaneous transmitter release at developing neuromuscular synapses. Neuron. 1991 May;6(5):837–843. doi: 10.1016/0896-6273(91)90179-4. [DOI] [PubMed] [Google Scholar]
- Fu W. M. Potentiation by ATP of the postsynaptic acetylcholine response at developing neuromuscular synapses in Xenopus cell cultures. J Physiol. 1994 Jun 15;477(Pt 3):449–458. doi: 10.1113/jphysiol.1994.sp020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W. M. Potentiation of acetylcholine responses in Xenopus embryonic muscle cells by dibutyryl cAMP. Pflugers Arch. 1993 Dec;425(5-6):439–445. doi: 10.1007/BF00374870. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Bessis A., Changeux J. P. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu Rev Pharmacol Toxicol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Diamond I. Phosphorylation of acetylcholine receptor by endogenous membrane protein kinase in receptor-enriched membranes of Torpedo californica. Nature. 1977 Jun 9;267(5611):539–540. doi: 10.1038/267539a0. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., McGuinness T. L., Huganir R. L., Greengard P. Role of protein phosphorylation in neuronal signal transduction. FASEB J. 1989 Mar;3(5):1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Hopfield J. F., Tank D. W., Greengard P., Huganir R. L. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988 Dec 15;336(6200):677–680. doi: 10.1038/336677a0. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1130–1134. doi: 10.1073/pnas.80.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Miles K. Protein phosphorylation of nicotinic acetylcholine receptors. Crit Rev Biochem Mol Biol. 1989;24(3):183–215. doi: 10.3109/10409238909082553. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Honig M. G. Excitatory action of ATP on embryonic chick muscle. J Neurosci. 1986 Mar;6(3):681–690. doi: 10.1523/JNEUROSCI.06-03-00681.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B. Experimental approaches to understanding the role of protein phosphorylation in the regulation of neuronal function. Annu Rev Neurosci. 1983;6:493–525. doi: 10.1146/annurev.ne.06.030183.002425. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y., Saito M. Early cross-striation formation in twitching Xenopus myocytes in culture. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1978–1982. doi: 10.1073/pnas.85.6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Calcitonin gene-related peptide elevates cyclic AMP levels in chick skeletal muscle: possible neurotrophic role for a coexisting neuronal messenger. EMBO J. 1987 Apr;6(4):901–906. doi: 10.1002/j.1460-2075.1987.tb04836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J., Nakajima S., Nakajima Y., Takahashi T. Differential development of two classes of acetylcholine receptors in Xenopus muscle in culture. Science. 1984 Oct 5;226(4670):55–57. doi: 10.1126/science.6474189. [DOI] [PubMed] [Google Scholar]
- Lo Y. J., Poo M. M. Activity-dependent synaptic competition in vitro: heterosynaptic suppression of developing synapses. Science. 1991 Nov 15;254(5034):1019–1022. doi: 10.1126/science.1658939. [DOI] [PubMed] [Google Scholar]
- Lu B., Fu W. M., Greengard P., Poo M. M. Calcitonin gene-related peptide potentiates synaptic responses at developing neuromuscular junction. Nature. 1993 May 6;363(6424):76–79. doi: 10.1038/363076a0. [DOI] [PubMed] [Google Scholar]
- Middleton P., Jaramillo F., Schuetze S. M. Forskolin increases the rate of acetylcholine receptor desensitization at rat soleus endplates. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4967–4971. doi: 10.1073/pnas.83.13.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P., Rubin L. L., Schuetze S. M. Desensitization of acetylcholine receptors in rat myotubes is enhanced by agents that elevate intracellular cAMP. J Neurosci. 1988 Sep;8(9):3405–3412. doi: 10.1523/JNEUROSCI.08-09-03405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles K., Greengard P., Huganir R. L. Calcitonin gene-related peptide regulates phosphorylation of the nicotinic acetylcholine receptor in rat myotubes. Neuron. 1989 May;2(5):1517–1524. doi: 10.1016/0896-6273(89)90198-0. [DOI] [PubMed] [Google Scholar]
- Mulle C., Benoit P., Pinset C., Roa M., Changeux J. P. Calcitonin gene-related peptide enhances the rate of desensitization of the nicotinic acetylcholine receptor in cultured mouse muscle cells. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5728–5732. doi: 10.1073/pnas.85.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Owens J. L., Kullberg R. In vivo development of nicotinic acetylcholine receptor channels in Xenopus myotomal muscle. J Neurosci. 1989 Mar;9(3):1018–1028. doi: 10.1523/JNEUROSCI.09-03-01018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. B., Chen Q. M., de Biasi S., Zhu D. L. Development of calcitonin gene-related peptide (CGRP) immunoreactivity in relationship to the formation of neuromuscular junctions in Xenopus myotomal muscle. J Comp Neurol. 1989 Dec 22;290(4):533–543. doi: 10.1002/cne.902900408. [DOI] [PubMed] [Google Scholar]
- Qu Z. C., Moritz E., Huganir R. L. Regulation of tyrosine phosphorylation of the nicotinic acetylcholine receptor at the rat neuromuscular junction. Neuron. 1990 Mar;4(3):367–378. doi: 10.1016/0896-6273(90)90049-l. [DOI] [PubMed] [Google Scholar]
- Rohrbough J., Kidokoro Y. Changes in kinetics of acetylcholine receptor channels after initial expression in Xenopus myocyte culture. J Physiol. 1990 Jun;425:245–269. doi: 10.1113/jphysiol.1990.sp018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A., Rapuano M., Prives J. Induction of phosphorylation and cell surface redistribution of acetylcholine receptors by phorbol ester and carbamylcholine in cultured chick muscle cells. J Cell Biol. 1988 Sep;107(3):1139–1145. doi: 10.1083/jcb.107.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safran A., Sagi-Eisenberg R., Neumann D., Fuchs S. Phosphorylation of the acetylcholine receptor by protein kinase C and identification of the phosphorylation site within the receptor delta subunit. J Biol Chem. 1987 Aug 5;262(22):10506–10510. [PubMed] [Google Scholar]
- Sanes D. H., Poo M. M. In vitro analysis of position- and lineage-dependent selectivity in the formation of neuromuscular synapses. Neuron. 1989 Mar;2(3):1237–1244. doi: 10.1016/0896-6273(89)90308-5. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Merlie J. P., Lawrence J. C., Jr Regulation of phosphorylation of nicotinic acetylcholine receptors in mouse BC3H1 myocytes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6601–6605. doi: 10.1073/pnas.84.18.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichberg V. I., Sobel A., Changeux J. P. In vitro phosphorylation of the acetylcholine receptor. Nature. 1977 Jun 9;267(5611):540–542. doi: 10.1038/267540a0. [DOI] [PubMed] [Google Scholar]
- Uchida S., Yamamoto H., Iio S., Matsumoto N., Wang X. B., Yonehara N., Imai Y., Inoki R., Yoshida H. Release of calcitonin gene-related peptide-like immunoreactive substance from neuromuscular junction by nerve excitation and its action on striated muscle. J Neurochem. 1990 Mar;54(3):1000–1003. doi: 10.1111/j.1471-4159.1990.tb02349.x. [DOI] [PubMed] [Google Scholar]
- Wallace B. G., Qu Z., Huganir R. L. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron. 1991 Jun;6(6):869–878. doi: 10.1016/0896-6273(91)90227-q. [DOI] [PubMed] [Google Scholar]
- Xie Z. P., Poo M. M. Initial events in the formation of neuromuscular synapse: rapid induction of acetylcholine release from embryonic neuron. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee G. H., Huganir R. L. Determination of the sites of cAMP-dependent phosphorylation on the nicotinic acetylcholine receptor. J Biol Chem. 1987 Dec 5;262(34):16748–16753. [PubMed] [Google Scholar]
- Zimmermann H. Turnover of adenine nucleotides in cholinergic synaptic vesicles of the Torpedo electric organ. Neuroscience. 1978;3(9):827–836. doi: 10.1016/0306-4522(78)90035-0. [DOI] [PubMed] [Google Scholar]