Abstract
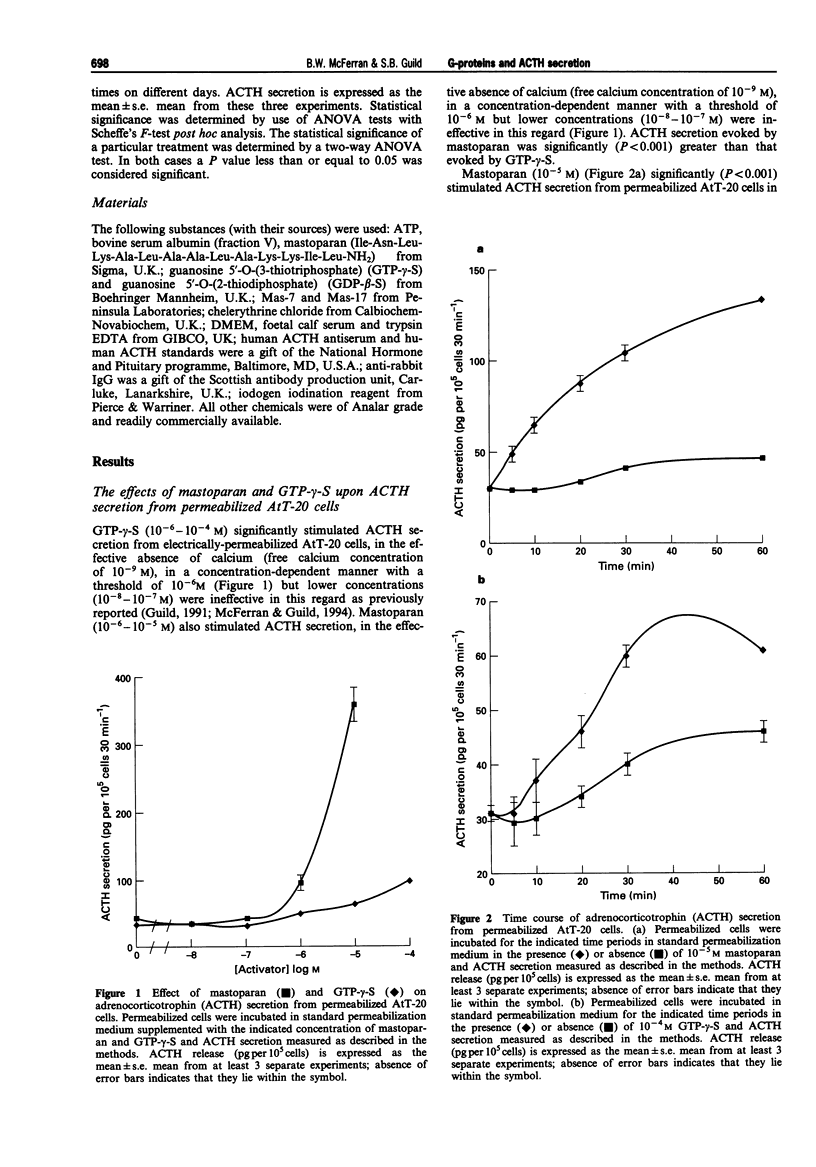
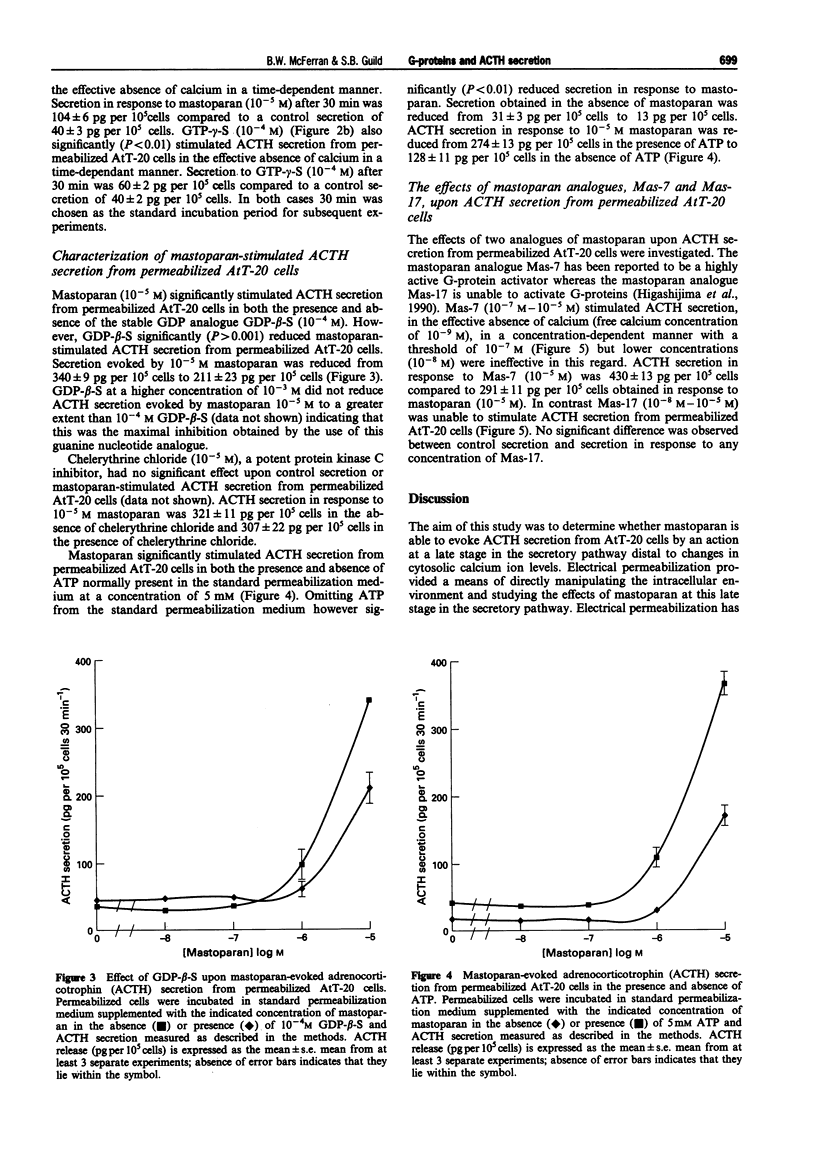
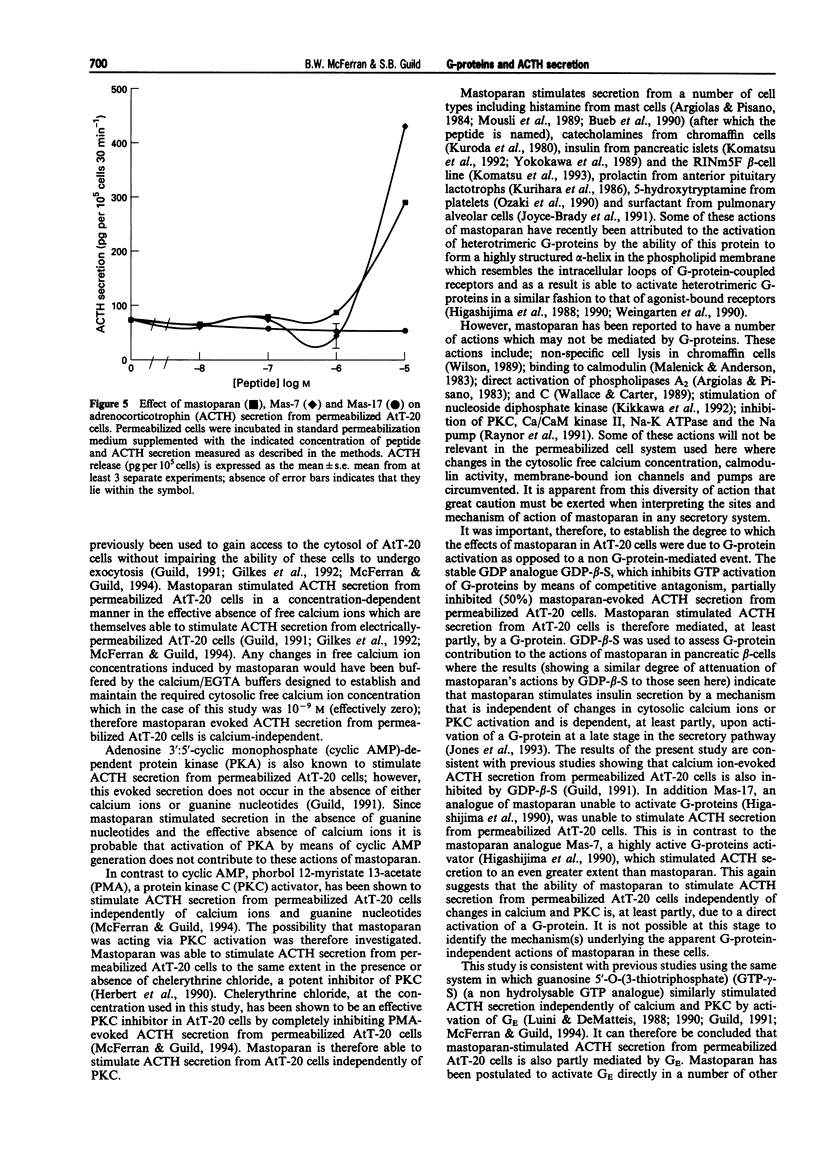
1. The mouse AtT-20/D16-16 anterior pituitary tumour cell line was used as a model system for the study of the effects of mastoparan upon the late stages of the adrenocorticotrophin (ACTH) secretory pathway. 2. Mastoparan (10(-8)-10(-5) M), an activator of heterotrimeric guanosine 5'-triphosphate binding proteins (G-proteins), stimulated ACTH secretion from electrically-permeabilized AtT-20 cells in a concentration-dependent manner in the effective absence of calcium ions with a threshold of 10(-6) M. Guanosine 5'-O-(3-thiotriphosphate) (GTP-gamma-S) (10(-8)-10(-4) M) also stimulated ACTH secretion from electrically-permeabilized AtT-20 cells in a concentration-dependent manner in the effective absence of calcium ions with a threshold of 10(-6) M. This GTP-gamma-S-evoked secretion is consistent with previous studies which demonstrated that a G-protein, termed GE, mediates calcium evoked ACTH secretion from AtT-20 cells. GTP-gamma-S-evoked secretion however was not as great as that obtained in response to mastoparan. 3. Both mastoparan (10(-5) M) and GTP-gamma-S (10(-4) M) stimulated ACTH secretion from electrically-permeabilized AtT20 cells in a time-dependent manner. A time of 30 min was adopted as the standard incubation period for the study of both mastoparan and GTP-gamma-S-stimulated ACTH secretion from permeabilized AtT-20 cells. 4. Mastoparan (10(-8)-10(-5) M) stimulated ACTH secretion from permeabilized AtT-20 cells to the same extent in the presence and absence of the protein kinase C (PKC) inhibitor, chelerythrine chloride (10(-5) M).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoni F. A., Holmes M. C., Jones M. T. Oxytocin as well as vasopressin potentiate ovine CRF in vitro. Peptides. 1983 Jul-Aug;4(4):411–415. doi: 10.1016/0196-9781(83)90041-4. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Pisano J. J. Facilitation of phospholipase A2 activity by mastoparans, a new class of mast cell degranulating peptides from wasp venom. J Biol Chem. 1983 Nov 25;258(22):13697–13702. [PubMed] [Google Scholar]
- Aridor M., Traub L. M., Sagi-Eisenberg R. Exocytosis in mast cells by basic secretagogues: evidence for direct activation of GTP-binding proteins. J Cell Biol. 1990 Sep;111(3):909–917. doi: 10.1083/jcb.111.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueb J. L., Mousli M., Bronner C., Rouot B., Landry Y. Activation of Gi-like proteins, a receptor-independent effect of kinins in mast cells. Mol Pharmacol. 1990 Dec;38(6):816–822. [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Low molecular mass GTP-binding proteins of adrenal chromaffin cells are present on the secretory granule. FEBS Lett. 1989 Mar 13;245(1-2):122–126. doi: 10.1016/0014-5793(89)80204-2. [DOI] [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Südhof T. C., Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991 Jan 3;349(6304):79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gilkes A. F., MacKay K. B., Cramb G., Guild S. B. Atrial natriuretic peptide effects in AtT-20 pituitary tumour cells. Mol Cell Endocrinol. 1992 Nov;89(1-2):39–45. doi: 10.1016/0303-7207(92)90209-o. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. GE: a GTP-binding protein mediating exocytosis. Annu Rev Physiol. 1990;52:591–606. doi: 10.1146/annurev.ph.52.030190.003111. [DOI] [PubMed] [Google Scholar]
- Guild S. Effects of adenosine 3':5'-cyclic monophosphate and guanine nucleotides on calcium-evoked ACTH release from electrically permeabilized AtT-20 cells. Br J Pharmacol. 1991 Sep;104(1):117–122. doi: 10.1111/j.1476-5381.1991.tb12394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Augereau J. M., Gleye J., Maffrand J. P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990 Nov 15;172(3):993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Burnier J., Ross E. M. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J Biol Chem. 1990 Aug 25;265(24):14176–14186. [PubMed] [Google Scholar]
- Higashijima T., Uzu S., Nakajima T., Ross E. M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem. 1988 May 15;263(14):6491–6494. [PubMed] [Google Scholar]
- Jones P. M., Mann F. M., Persaud S. J., Wheeler-Jones C. P. Mastoparan stimulates insulin secretion from pancreatic beta-cells by effects at a late stage in the secretory pathway. Mol Cell Endocrinol. 1993 Jul;94(1):97–103. doi: 10.1016/0303-7207(93)90056-p. [DOI] [PubMed] [Google Scholar]
- Joyce-Brady M., Rubins J. B., Panchenko M. P., Bernardo J., Steele M. P., Kolm L., Simons E. R., Dickey B. F. Mechanisms of mastoparan-stimulated surfactant secretion from isolated pulmonary alveolar type 2 cells. J Biol Chem. 1991 Apr 15;266(11):6859–6865. [PubMed] [Google Scholar]
- Kikkawa S., Takahashi K., Takahashi K., Shimada N., Ui M., Kimura N., Katada T. Activation of nucleoside diphosphate kinase by mastoparan, a peptide isolated from wasp venom. FEBS Lett. 1992 Jul 6;305(3):237–240. doi: 10.1016/0014-5793(92)80676-8. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986 Mar 15;234(3):497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Aizawa T., Yokokawa N., Sato Y., Okada N., Takasu N., Yamada T. Mastoparan-induced hormone release from rat pancreatic islets. Endocrinology. 1992 Jan;130(1):221–228. doi: 10.1210/endo.130.1.1727698. [DOI] [PubMed] [Google Scholar]
- Komatsu M., McDermott A. M., Gillison S. L., Sharp G. W. Mastoparan stimulates exocytosis at a Ca(2+)-independent late site in stimulus-secretion coupling. Studies with the RINm5F beta-cell line. J Biol Chem. 1993 Nov 5;268(31):23297–23306. [PubMed] [Google Scholar]
- Kurihara H., Kitajima K., Senda T., Fujita H., Nakajima T. Multigranular exocytosis induced by phospholipase A2-activators, melittin and mastoparan, in rat anterior pituitary cells. Cell Tissue Res. 1986;243(2):311–316. doi: 10.1007/BF00251045. [DOI] [PubMed] [Google Scholar]
- Luini A., De Matteis M. A. Dual regulation of ACTH secretion by guanine nucleotides in permeabilized AtT-20 cells. Cell Mol Neurobiol. 1988 Mar;8(1):129–138. doi: 10.1007/BF00712918. [DOI] [PubMed] [Google Scholar]
- Luini A., De Matteis M. A. Evidence that receptor-linked G protein inhibits exocytosis by a post-second-messenger mechanism in AtT-20 cells. J Neurochem. 1990 Jan;54(1):30–38. doi: 10.1111/j.1471-4159.1990.tb13279.x. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. High affinity binding of the mastoparans by calmodulin. Biochem Biophys Res Commun. 1983 Jul 18;114(1):50–56. doi: 10.1016/0006-291x(83)91592-9. [DOI] [PubMed] [Google Scholar]
- McFerran B. W., Guild S. B. Effects of protein kinase C activators upon the late stages of the ACTH secretory pathway of AtT-20 cells. Br J Pharmacol. 1994 Sep;113(1):171–178. doi: 10.1111/j.1476-5381.1994.tb16190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousli M., Bronner C., Bueb J. L., Tschirhart E., Gies J. P., Landry Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J Pharmacol Exp Ther. 1989 Jul;250(1):329–335. [PubMed] [Google Scholar]
- Ngsee J. K., Fleming A. M., Scheller R. H. A rab protein regulates the localization of secretory granules in AtT-20 cells. Mol Biol Cell. 1993 Jul;4(7):747–756. doi: 10.1091/mbc.4.7.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki Y., Matsumoto Y., Yatomi Y., Higashihara M., Kariya T., Kume S. Mastoparan, a wasp venom, activates platelets via pertussis toxin-sensitive GTP-binding proteins. Biochem Biophys Res Commun. 1990 Jul 31;170(2):779–785. doi: 10.1016/0006-291x(90)92159-w. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R. GTP-binding proteins in intracellular transport. Trends Cell Biol. 1992 Feb;2(2):41–46. doi: 10.1016/0962-8924(92)90161-f. [DOI] [PubMed] [Google Scholar]
- Raynor R. L., Zheng B., Kuo J. F. Membrane interactions of amphiphilic polypeptides mastoparan, melittin, polymyxin B, and cardiotoxin. Differential inhibition of protein kinase C, Ca2+/calmodulin-dependent protein kinase II and synaptosomal membrane Na,K-ATPase, and Na+ pump and differentiation of HL60 cells. J Biol Chem. 1991 Feb 15;266(5):2753–2758. [PubMed] [Google Scholar]
- Reisine T. Somatostatin desensitization: loss of the ability of somatostatin to inhibit cyclic AMP accumulation and adrenocorticotropin hormone release. J Pharmacol Exp Ther. 1984 Apr;229(1):14–20. [PubMed] [Google Scholar]
- Taylor C. W. The role of G proteins in transmembrane signalling. Biochem J. 1990 Nov 15;272(1):1–13. doi: 10.1042/bj2720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutant M., Aunis D., Bockaert J., Homburger V., Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987 May 11;215(2):339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- Wallace M. A., Carter H. R. Effects of the wasp venom peptide, mastoparan, on a phosphoinositide-specific phospholipase C purified from rabbit brain membranes. Biochim Biophys Acta. 1989 Dec 18;1006(3):311–316. doi: 10.1016/0005-2760(89)90018-0. [DOI] [PubMed] [Google Scholar]
- Weingarten R., Ransnäs L., Mueller H., Sklar L. A., Bokoch G. M. Mastoparan interacts with the carboxyl terminus of the alpha subunit of Gi. J Biol Chem. 1990 Jul 5;265(19):11044–11049. [PubMed] [Google Scholar]
- Wheeler-Jones C. P., Saermark T., Kakkar V. V., Authi K. S. Mastoparan promotes exocytosis and increases intracellular cyclic AMP in human platelets. Evidence for the existence of a Ge-like mechanism of secretion. Biochem J. 1992 Jan 15;281(Pt 2):465–472. doi: 10.1042/bj2810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. P. Effects of mastoparan on catecholamine release from chromaffin cells. FEBS Lett. 1989 Apr 24;247(2):239–241. doi: 10.1016/0014-5793(89)81343-2. [DOI] [PubMed] [Google Scholar]
- Yokokawa N., Komatsu M., Takeda T., Aizawa T., Yamada T. Mastoparan, a wasp venom, stimulates insulin release by pancreatic islets through pertussis toxin sensitive GTP-binding protein. Biochem Biophys Res Commun. 1989 Feb 15;158(3):712–716. doi: 10.1016/0006-291x(89)92779-4. [DOI] [PubMed] [Google Scholar]


