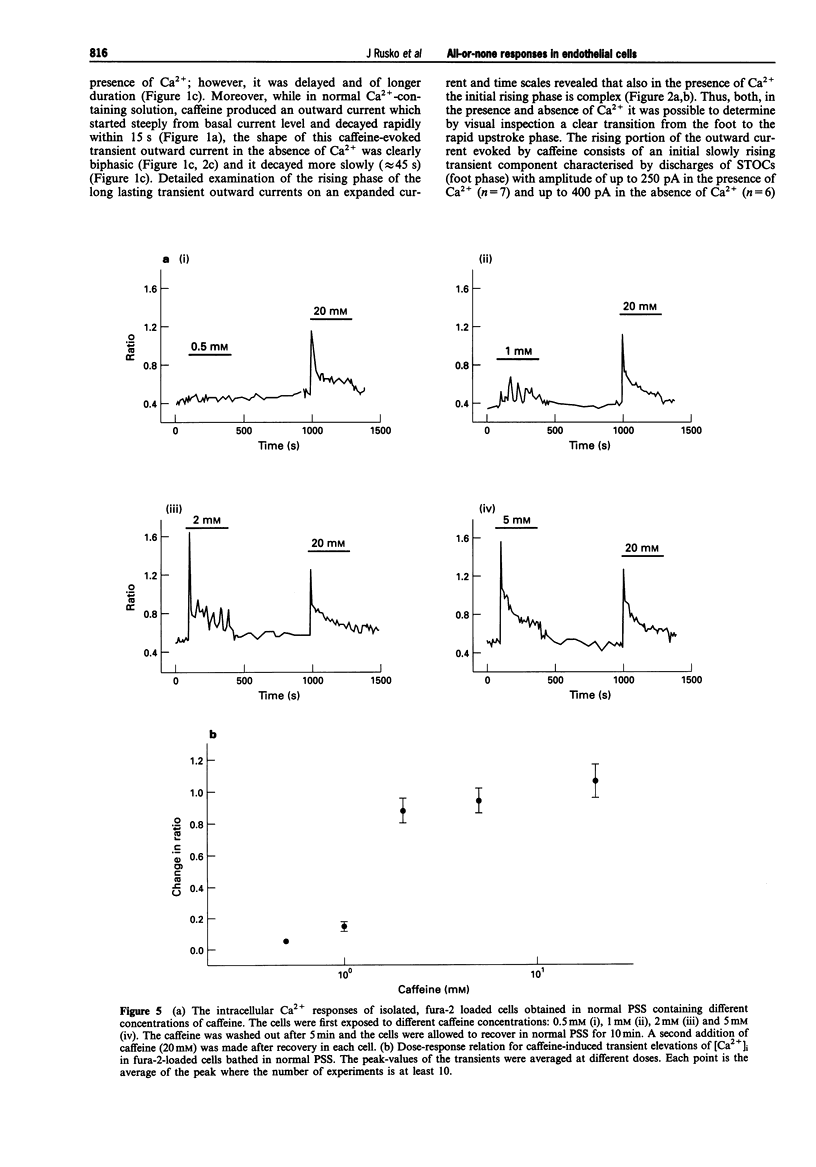
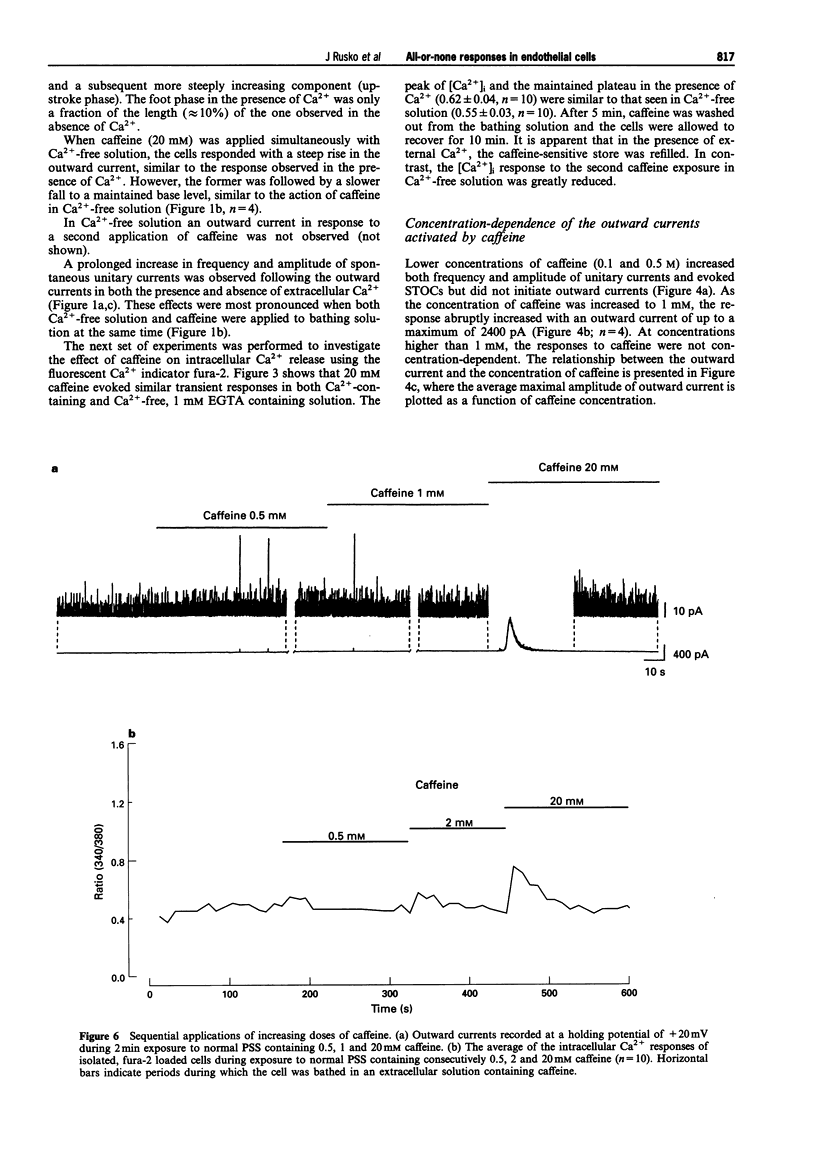
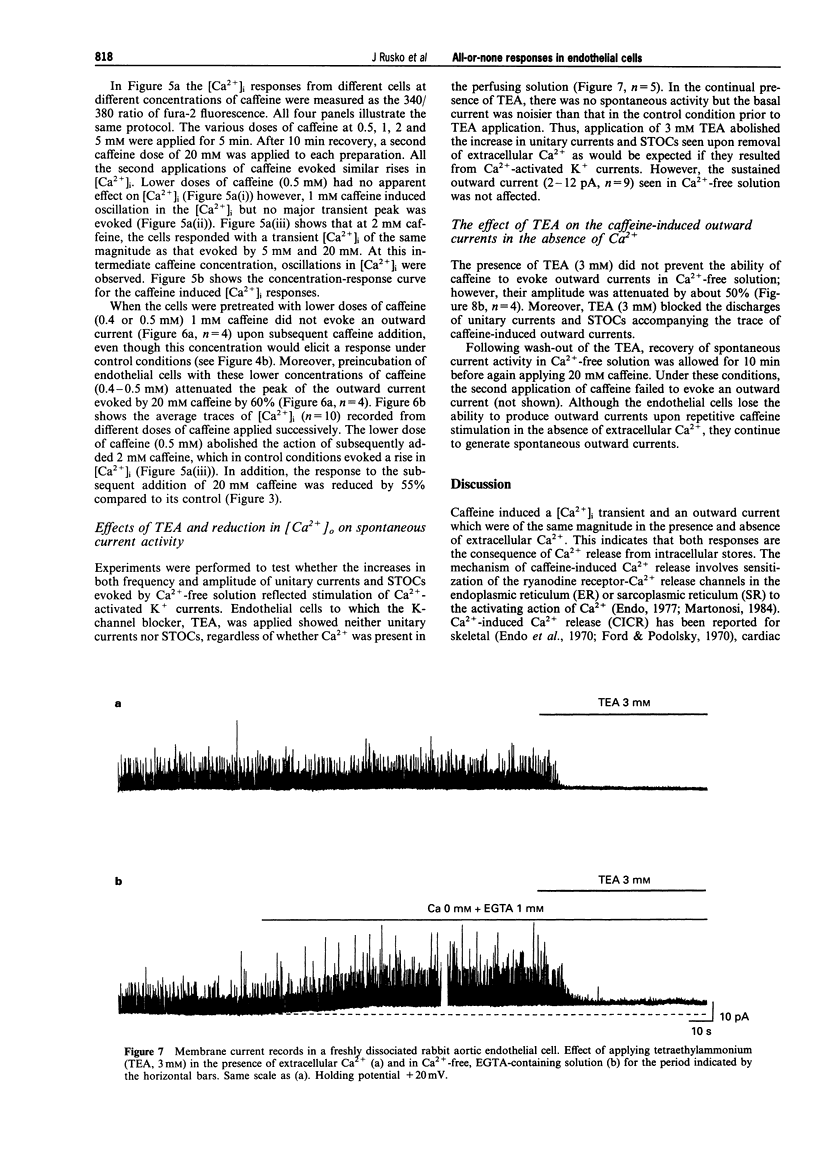
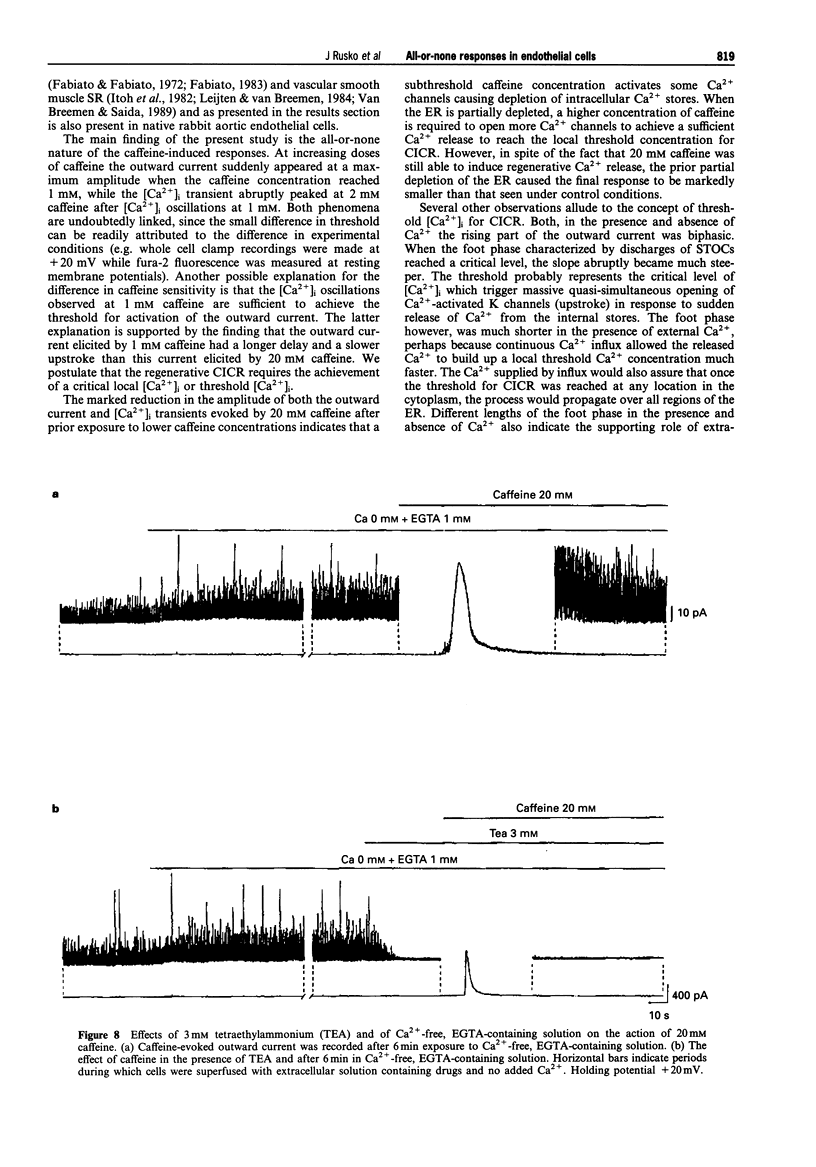
Abstract
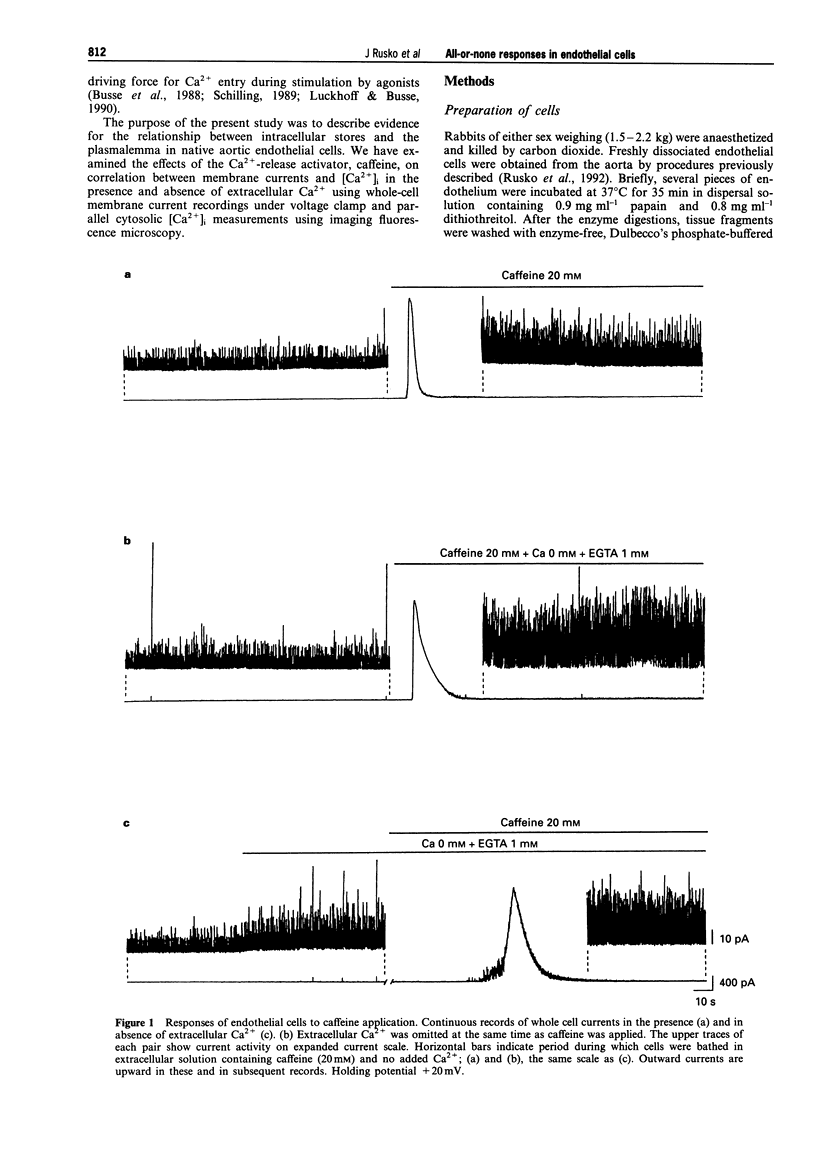
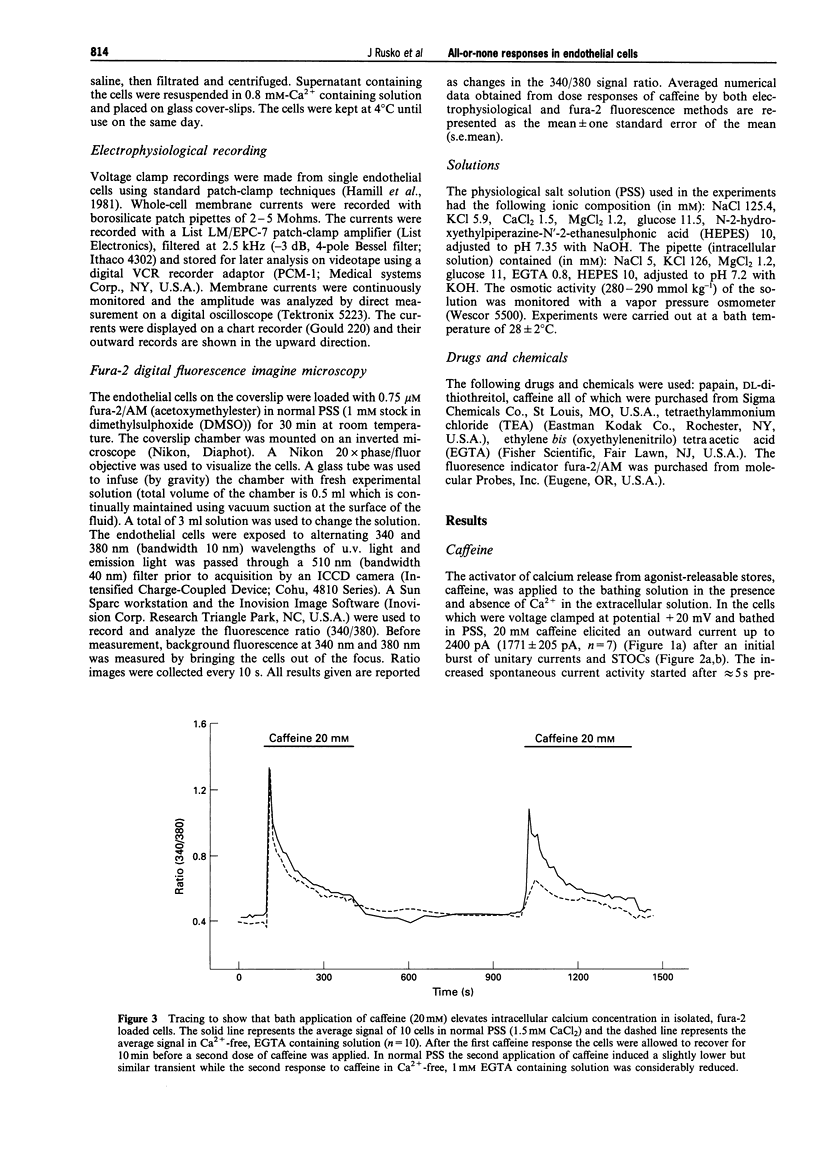
1. Single native aortic endothelial cells obtained by enzymatic dispersion of the rabbit aortic endothelium were held under voltage clamp using patch pipette and whole-cell membrane currents were measured. In parallel experiments performed on cells from the same batches, the free internal calcium concentration, [Ca2+]i, in the cell was estimated by use of the Ca(2+)-sensitive fluorescent dye, fura-2. 2. Caffeine (20 mM) applied to the cell evoked an outward current and an initial peak in [Ca2+]i followed by a lower sustained rise (plateau). Ca(2+)-free, EGTA-containing solution applied outside the cells did not reduce these responses. 3. Following caffeine stimulation there was a biphasic rising phase of outward current both in the presence and absence of extracellular Ca2+. 4. Application of graded doses of caffeine revealed all-or-none type responses of both the outward current and the rise in [Ca2+]i. 5. Preincubation with lower doses of caffeine reduced the magnitude of both the outward current and the [Ca2+]i transient evoked by 20 mM caffeine. 6. Tetraethylammonium (3 mM) applied to the bathing solution blocked unitary and spontaneous transient outward currents (STOCs) stimulated by Ca(2+)-free solution, but only reduced the outward current evoked by caffeine (20 mM). 7. In conclusion, our results reveal the all-or-none nature of Ca2+ release from the endoplasmic reticulum (ER) in native aortic endothelial cells. Lower concentrations of caffeine (0.4-0.5 mM) may deplete intracellular Ca2+ stores. Extracellular Ca2+ is not necessary for maintaining the activity of spontaneous and caffeine-induced outward currents in native aortic endothelial cells. Spontaneous outward currents are believed to represent the sporadic release of calcium from store sites independent of both extracellular Ca2+ and the caffeine-sensitive Ca2+ stores which stimulate the outward current.
Full text
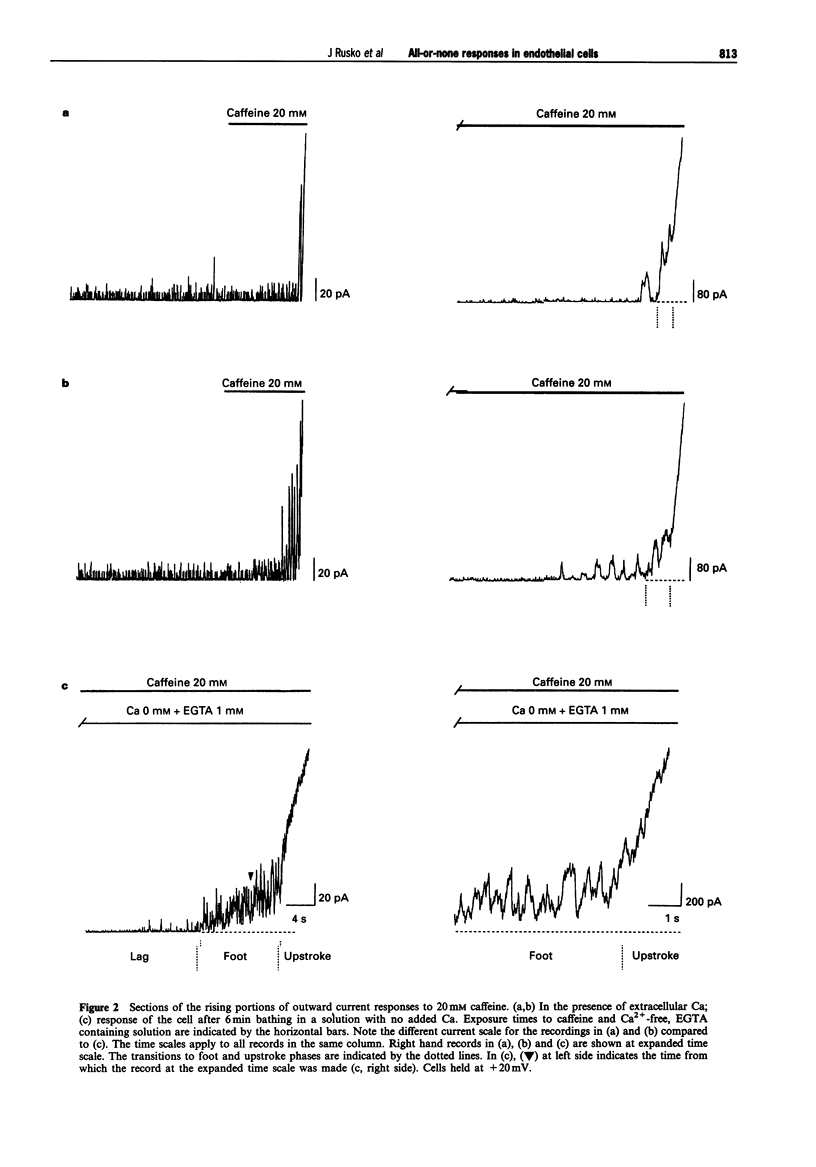
PDF
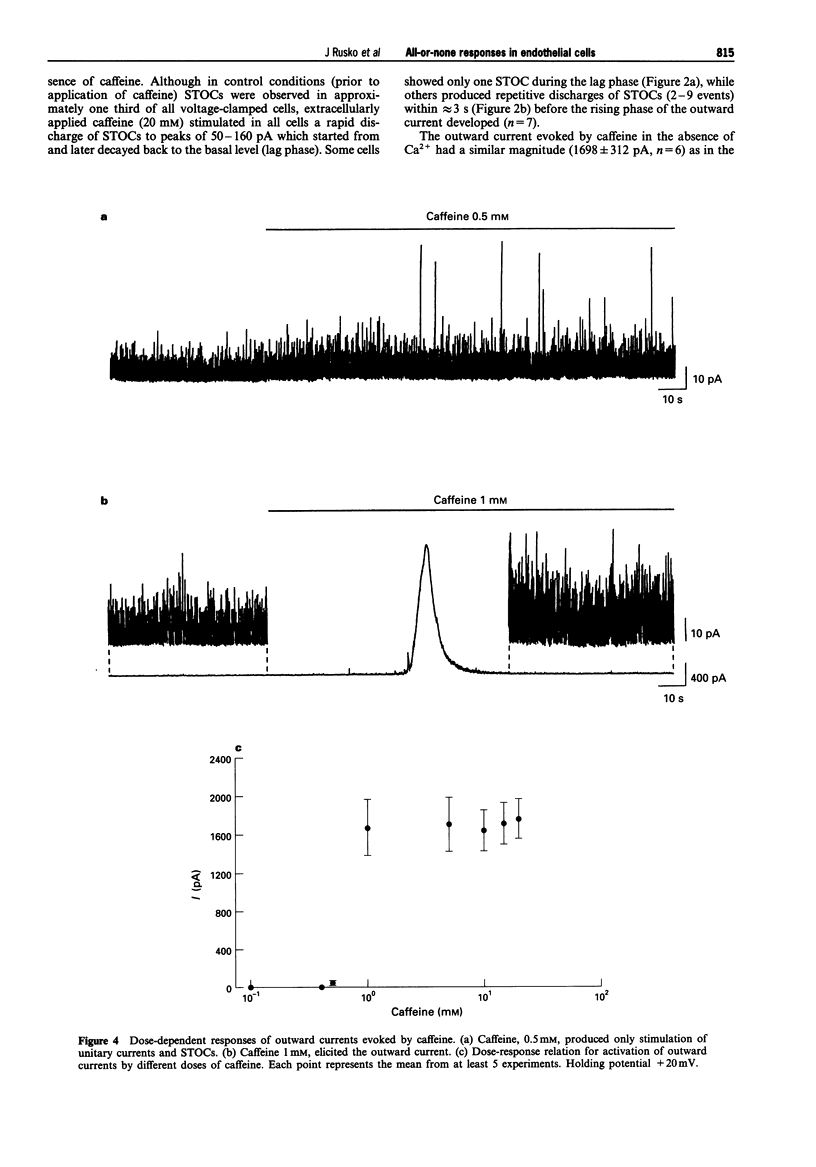


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan K. W., Martin W. Bradykinin induces elevations of cytosolic calcium through mobilisation of intracellular and extracellular pools in bovine aortic endothelial cells. Br J Pharmacol. 1991 Jan;102(1):35–40. doi: 10.1111/j.1476-5381.1991.tb12128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Campbell D. L., Strauss H. C., Whorton A. R. Voltage dependence of bovine pulmonary artery endothelial cell function. J Mol Cell Cardiol. 1991 Feb;23 (Suppl 1):133–144. doi: 10.1016/0022-2828(91)90032-h. [DOI] [PubMed] [Google Scholar]
- Chen G., Cheung D. W. Pharmacological distinction of the hyperpolarization response to caffeine and acetylcholine in guinea-pig coronary endothelial cells. Eur J Pharmacol. 1992 Nov 13;223(1):33–38. doi: 10.1016/0014-2999(92)90815-l. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972 Sep;31(3):293–307. doi: 10.1161/01.res.31.3.293. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Freay A., Johns A., Adams D. J., Ryan U. S., Van Breemen C. Bradykinin and inositol 1,4,5-trisphosphate-stimulated calcium release from intracellular stores in cultured bovine endothelial cells. Pflugers Arch. 1989 Aug;414(4):377–384. doi: 10.1007/BF00585046. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Johns A., Rubanyi G. M., van Breemen C. Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990 Feb 15;265(5):2613–2619. [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Martonosi A. N. Mechanisms of Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Physiol Rev. 1984 Oct;64(4):1240–1320. doi: 10.1152/physrev.1984.64.4.1240. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Adams D. J., van Breemen C. Synchronized oscillations in cytoplasmic free calcium concentration in confluent bradykinin-stimulated bovine pulmonary artery endothelial cell monolayers. J Biol Chem. 1989 Jan 5;264(1):6–9. [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Elliott S. J. Ca2+ signaling mechanisms of vascular endothelial cells and their role in oxidant-induced endothelial cell dysfunction. Am J Physiol. 1992 Jun;262(6 Pt 2):H1617–H1630. doi: 10.1152/ajpheart.1992.262.6.H1617. [DOI] [PubMed] [Google Scholar]
- Zhang A., Cheng T. P., Altura B. T., Altura B. M. Mg2+ and caffeine-induced intracellular Ca2+ release in human vascular endothelial cells. Br J Pharmacol. 1993 Jun;109(2):291–292. doi: 10.1111/j.1476-5381.1993.tb13568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]



