Abstract
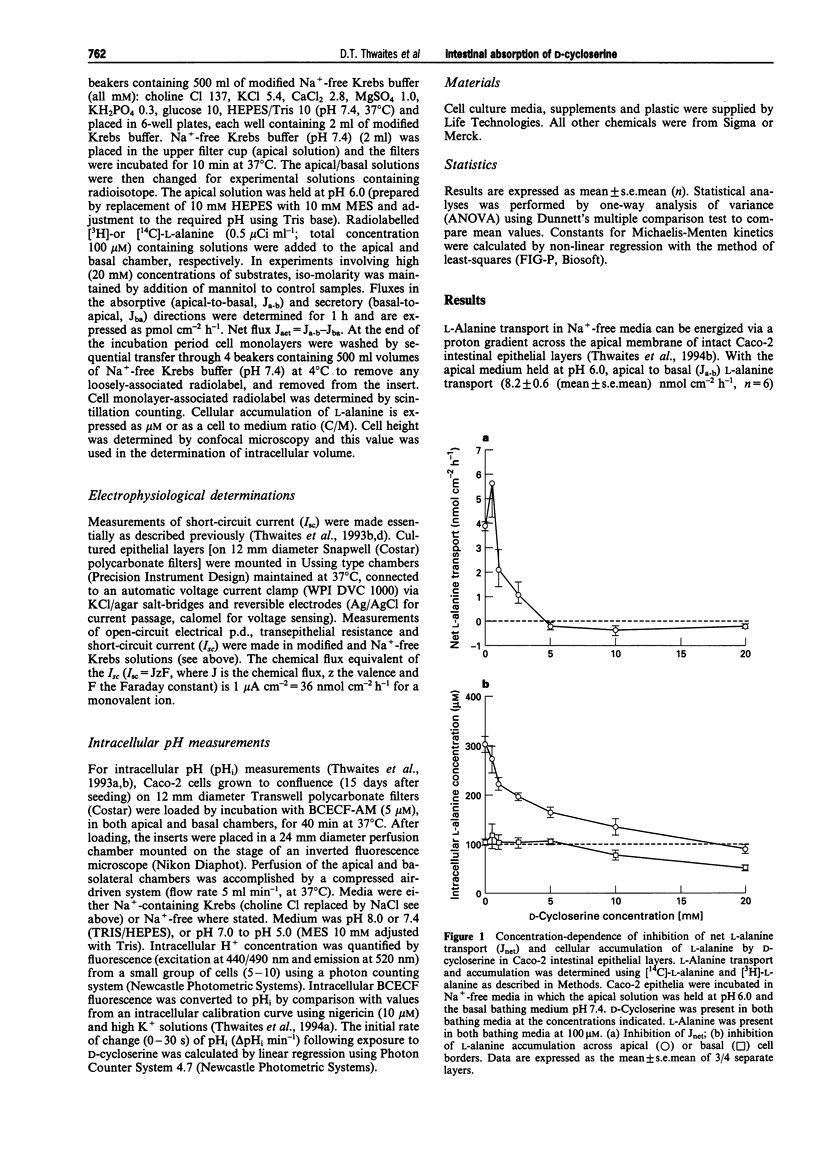
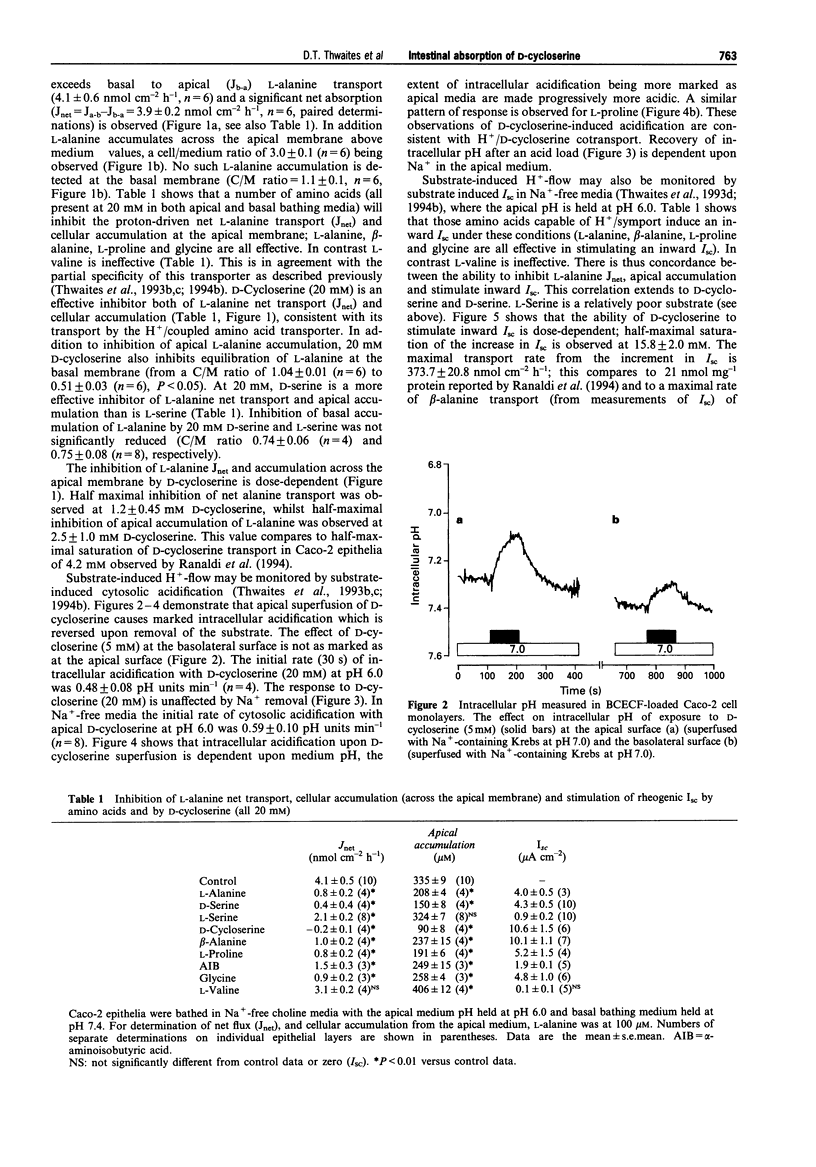
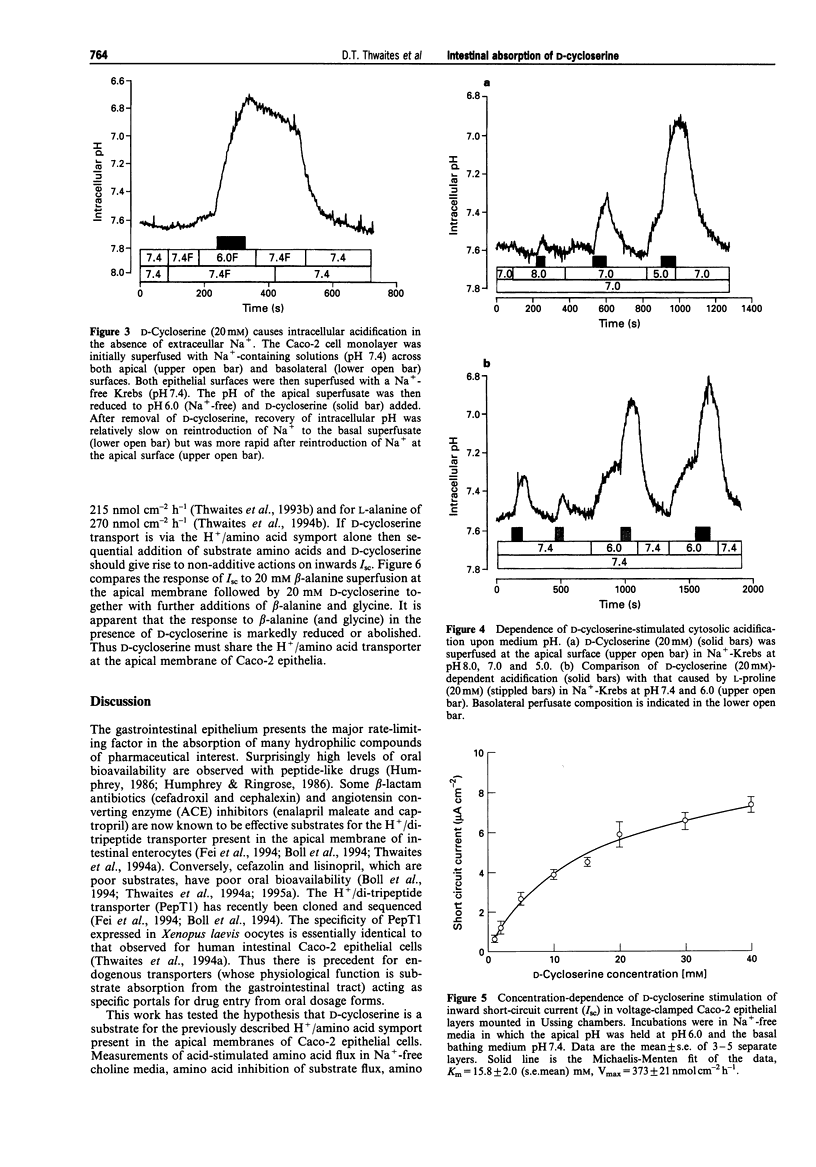
1. The ability of D-cycloserine to act as a substrate for H+/amino acid symport has been tested in epithelial layers of Caco-2 human intestinal cells. 2. In Na(+)-free media with the apical bathing media held at pH 6.0, D-cycloserine (20 mM) is an effective inhibitor of net transepithelial transport (Jnet) of L-alanine (100 microM) and its accumulation (across the apical membrane) in a similar manner to amino acid substrates (L-alanine, beta-alanine, L-proline and glycine). In contrast L-valine was ineffective as an inhibitor for H+/amino acid symport. Both inhibition of L-alanine Jnet and its accumulation by D-cycloserine were dose-dependent, maximal inhibition being achieved by 5-10 mM. 3. Both D-cycloserine and known substrates for H+/amino acid symport stimulated an inward short circuit current (Isc) when voltage-clamped monolayers of Caco-2 epithelia, mounted in Ussing chambers, were exposed to apical substrate in Na(+)-free media, with apical pH held at 6.0. The D-cycloserine dependent increase in Isc was dose-dependent with an apparent Km = 15.8 +/- 2.0 (mean +/- s.e. mean) mM, and Vmax = 373 +/- 21 nmol cm-2h-1. 4. D-Cycloserine (20 mM) induced a prompt acidification of Caco-2 cell cytosol when superfused at the apical surface in both Na+ and Na(+)-free conditions. Cytosolic acidification in response to D-cycloserine was dependent upon superfusate pH, being attenuated at pH 8 and enhanced in acidic media. 5. The increment in Isc with 20 mM D-cycloserine was non-additive with other amino acid substrates for H+/amino acid symport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker G. A., Ellory J. C. The identification of neutral amino acid transport systems. Exp Physiol. 1990 Jan;75(1):3–26. doi: 10.1113/expphysiol.1990.sp003382. [DOI] [PubMed] [Google Scholar]
- Boll M., Markovich D., Weber W. M., Korte H., Daniel H., Murer H. Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, beta-lactam antibiotics and ACE-inhibitors. Pflugers Arch. 1994 Nov;429(1):146–149. doi: 10.1007/BF02584043. [DOI] [PubMed] [Google Scholar]
- Fei Y. J., Kanai Y., Nussberger S., Ganapathy V., Leibach F. H., Romero M. F., Singh S. K., Boron W. F., Hediger M. A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994 Apr 7;368(6471):563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Ganapathy, Leibach F. H. Is intestinal peptide transport energized by a proton gradient? Am J Physiol. 1985 Aug;249(2 Pt 1):G153–G160. doi: 10.1152/ajpgi.1985.249.2.G153. [DOI] [PubMed] [Google Scholar]
- Humphrey M. J., Ringrose P. S. Peptides and related drugs: a review of their absorption, metabolism, and excretion. Drug Metab Rev. 1986;17(3-4):283–310. doi: 10.3109/03602538608998293. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Characteristics of cysteine uptake in intestinal basolateral membrane vesicles. Am J Physiol. 1984 Oct;247(4 Pt 1):G394–G401. doi: 10.1152/ajpgi.1984.247.4.G394. [DOI] [PubMed] [Google Scholar]
- Lucas M. L., Schneider W., Haberich F. J., Blair J. A. Direct measurement by pH-microelectrode of the pH microclimate in rat proximal jejunum. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):39–48. doi: 10.1098/rspb.1975.0150. [DOI] [PubMed] [Google Scholar]
- McEwan G. T., Daniel H., Fett C., Burgess M. N., Lucas M. L. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc R Soc Lond B Biol Sci. 1988 Jul 22;234(1275):219–237. doi: 10.1098/rspb.1988.0045. [DOI] [PubMed] [Google Scholar]
- Munck B. G., Munck L. K., Rasmussen S. N., Polache A. Specificity of the imino acid carrier in rat small intestine. Am J Physiol. 1994 Apr;266(4 Pt 2):R1154–R1161. doi: 10.1152/ajpregu.1994.266.4.R1154. [DOI] [PubMed] [Google Scholar]
- Quartermain D., Mower J., Rafferty M. F., Herting R. L., Lanthorn T. H. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994 May 12;257(1-2):7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- Ranaldi G., Islam K., Sambuy Y. D-cycloserine uses an active transport mechanism in the human intestinal cell line Caco 2. Antimicrob Agents Chemother. 1994 Jun;38(6):1239–1245. doi: 10.1128/aac.38.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotwell M. A., Jayme D. W., Kilberg M. S., Oxender D. L. Neutral amino acid transport systems in Chinese hamster ovary cells. J Biol Chem. 1981 Jun 10;256(11):5422–5427. [PubMed] [Google Scholar]
- Stevens B. R., Kaunitz J. D., Wright E. M. Intestinal transport of amino acids and sugars: advances using membrane vesicles. Annu Rev Physiol. 1984;46:417–433. doi: 10.1146/annurev.ph.46.030184.002221. [DOI] [PubMed] [Google Scholar]
- Thwaites D. T., Brown C. D., Hirst B. H., Simmons N. L. H(+)-coupled dipeptide (glycylsarcosine) transport across apical and basal borders of human intestinal Caco-2 cell monolayers display distinctive characteristics. Biochim Biophys Acta. 1993 Sep 19;1151(2):237–245. doi: 10.1016/0005-2736(93)90108-c. [DOI] [PubMed] [Google Scholar]
- Thwaites D. T., Brown C. D., Hirst B. H., Simmons N. L. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H(+)-coupled carriers at both apical and basal membranes. J Biol Chem. 1993 Apr 15;268(11):7640–7642. [PubMed] [Google Scholar]
- Thwaites D. T., Cavet M., Hirst B. H., Simmons N. L. Angiotensin-converting enzyme (ACE) inhibitor transport in human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 1995 Mar;114(5):981–986. doi: 10.1111/j.1476-5381.1995.tb13301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites D. T., Hirst B. H., Simmons N. L. Substrate specificity of the di/tripeptide transporter in human intestinal epithelia (Caco-2): identification of substrates that undergo H(+)-coupled absorption. Br J Pharmacol. 1994 Nov;113(3):1050–1056. doi: 10.1111/j.1476-5381.1994.tb17099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites D. T., McEwan G. T., Brown C. D., Hirst B. H., Simmons N. L. L-alanine absorption in human intestinal Caco-2 cells driven by the proton electrochemical gradient. J Membr Biol. 1994 Jun;140(2):143–151. doi: 10.1007/BF00232902. [DOI] [PubMed] [Google Scholar]
- Thwaites D. T., McEwan G. T., Cook M. J., Hirst B. H., Simmons N. L. H(+)-coupled (Na(+)-independent) proline transport in human intestinal (Caco-2) epithelial cell monolayers. FEBS Lett. 1993 Oct 25;333(1-2):78–82. doi: 10.1016/0014-5793(93)80378-8. [DOI] [PubMed] [Google Scholar]
- Thwaites D. T., McEwan G. T., Hirst B. H., Simmons N. L. H(+)-coupled alpha-methylaminoisobutyric acid transport in human intestinal Caco-2 cells. Biochim Biophys Acta. 1995 Mar 8;1234(1):111–118. doi: 10.1016/0005-2736(94)00268-t. [DOI] [PubMed] [Google Scholar]
- Weltman A. C., Rose D. N. Tuberculosis susceptibility patterns, predictors of multidrug resistance, and implications for initial therapeutic regimens at a New York City hospital. Arch Intern Med. 1994 Oct 10;154(19):2161–2167. [PubMed] [Google Scholar]



