Abstract
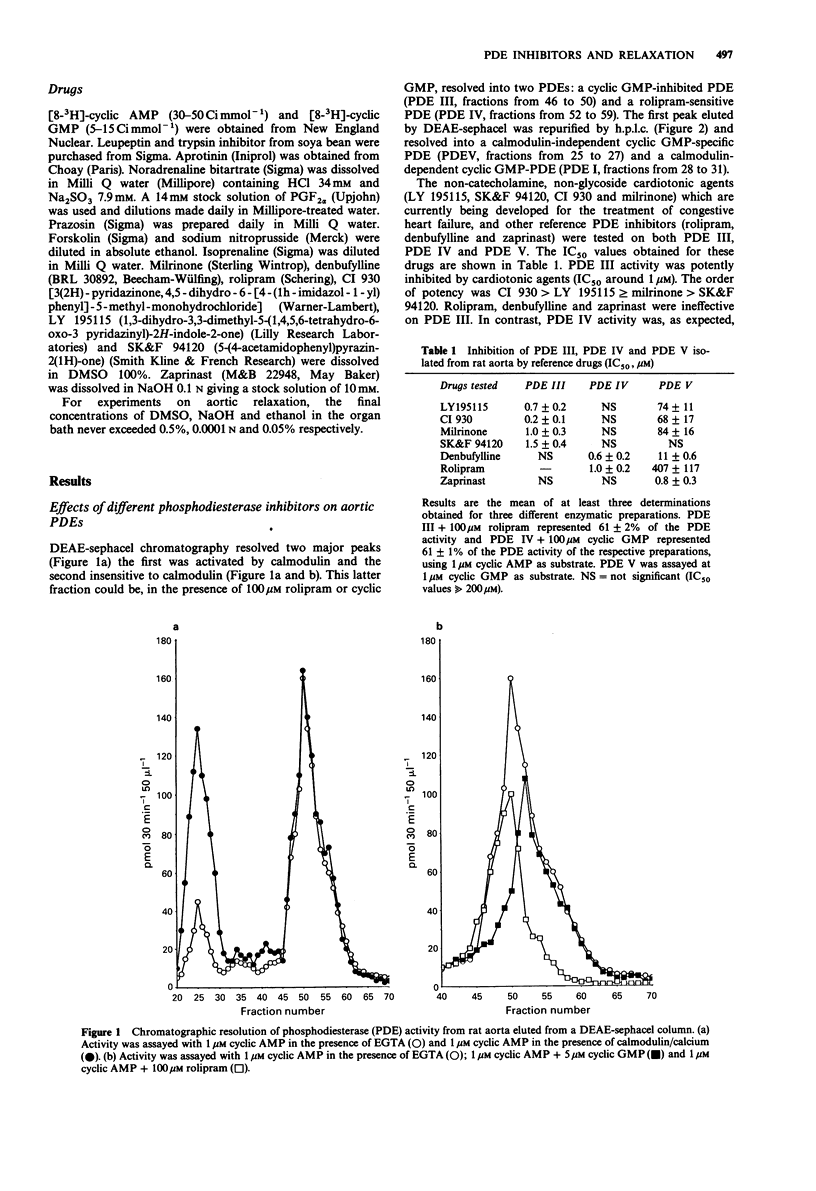
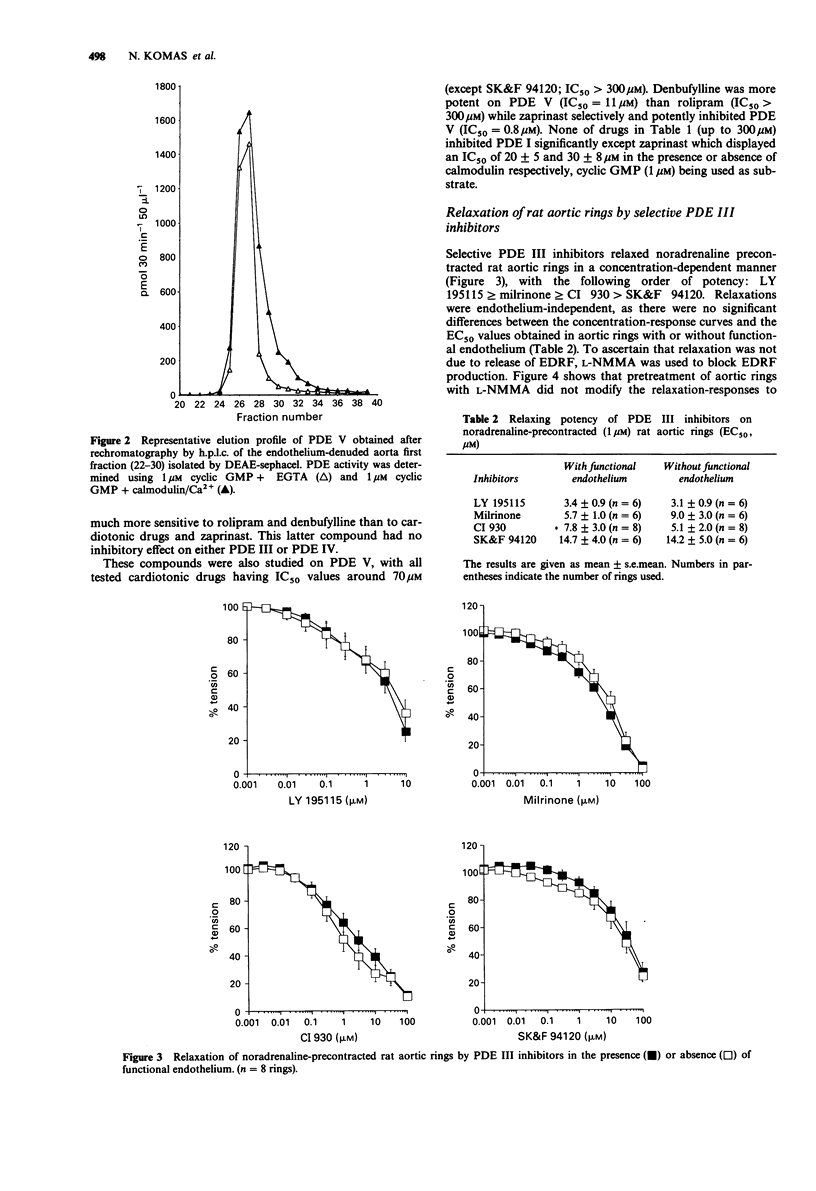
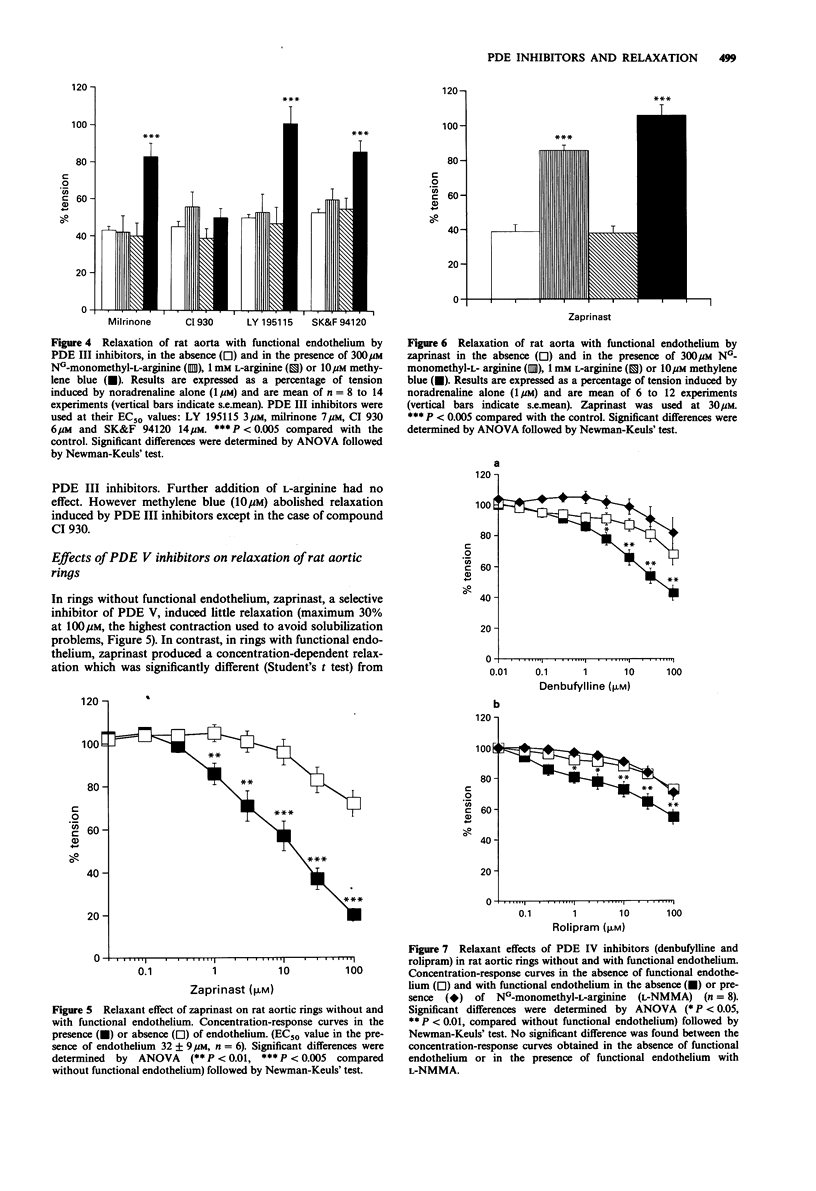
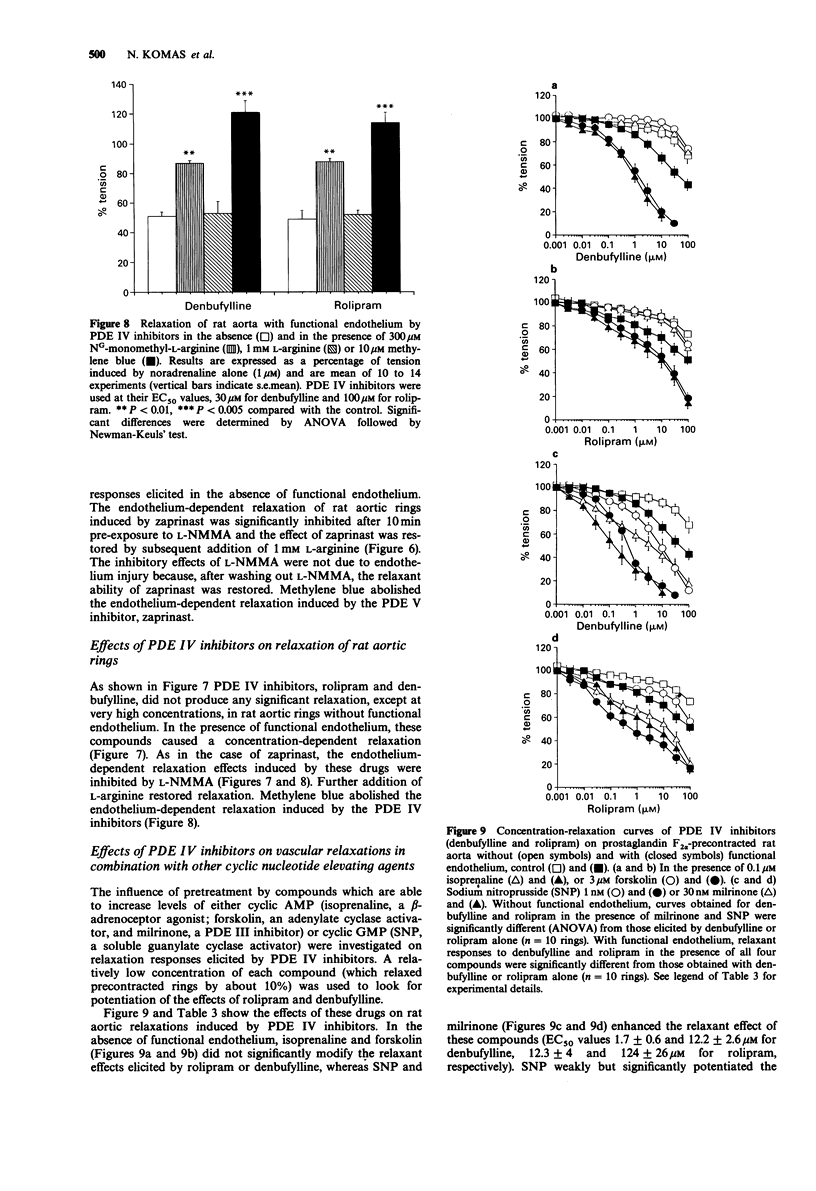
1. The effects of selective inhibitors of adenosine 3':5'-cyclic monophosphate (cyclic AMP) and guanosine 3':5'-cyclic monophosphate (cyclic GMP) phosphodiesterases (PDEs) were investigated on PDEs isolated from the rat aorta and on relaxation of noradrenaline (1 microM) precontracted rat aortic rings, with and without functional endothelium. 2. Four PDE forms were isolated by DEAE-sephacel chromatography from endothelium-denuded rat aorta: a calmodulin-activated PDE (PDE I) which hydrolyzed preferentially cyclic GMP, two cyclic AMP PDEs (PDE III and PDE IV) and one cyclic GMP-specific PDE (PDE V). The latter was selectively and potently inhibited by zaprinast. The two cyclic AMP PDEs were discriminated by specific inhibitors: one was inhibited by cyclic GMP (PDE III) and by new cardiotonic agents (milrinone, CI 930, LY 195115 and SK&F 94120); the other was inhibited by denbufylline and rolipram (PDE IV). None of these drugs significantly inhibited PDE I. 3. The PDE III inhibitors caused endothelium-independent relaxations of rat aortic rings with the following EC50 values (microM concentration producing 50% relaxation): LY 195115: 3.4, milrinone: 5.7, CI 930; 7.8, SK&F 94120: 14.7. Neither NG-monomethyl-L-arginine (L-NMMA, 300 microM), an inhibitor of the L-arginine-NO pathway, nor L-arginine (1 mM) modified the effect of PDE III inhibitors. However, methylene blue (10 microM) an inhibitor of soluble guanylate cyclase abolished relaxation induced by PDE III inhibitors except in the case of compound CI 930. 4. The specific PDE IV and PDE V inhibitors both produced endothelium-dependent relaxations which were inhibited by L-NMMA and by methylene blue (10 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alousi A. A., Stankus G. P., Stuart J. C., Walton L. H. Characterization of the cardiotonic effects of milrinone, a new and potent cardiac bipyridine, on isolated tissues from several animal species. J Cardiovasc Pharmacol. 1983 Sep-Oct;5(5):804–811. doi: 10.1097/00005344-198309000-00015. [DOI] [PubMed] [Google Scholar]
- Arimura H., Ikemoto Y. Action of enflurane on cholinergic transmission in identified Aplysia neurones. Br J Pharmacol. 1986 Nov;89(3):573–582. doi: 10.1111/j.1476-5381.1986.tb11158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Bär H. P. Cyclic nucleotides and smooth muscle. Adv Cyclic Nucleotide Res. 1974;4(0):195–237. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gristwood R. W., Eden R. J., Owen D. A., Taylor E. M. Pharmacological studies with SK&F 94120, a novel positive inotropic agent with vasodilator activity. J Pharm Pharmacol. 1986 Jun;38(6):452–459. doi: 10.1111/j.2042-7158.1986.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Chang M. L., Beavo J. A. Differential inhibition of cardiac cyclic nucleotide phosphodiesterase isozymes by cardiotonic drugs. Circulation. 1986 Mar;73(3 Pt 2):III109–III116. [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Hayes J. S., Pollock G. D., Wilson H., Bowling N., Robertson D. W. Pharmacology of LY175326: a potent cardiotonic agent with vasodilator activities. J Pharmacol Exp Ther. 1985 May;233(2):318–326. [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Ito M., Tanaka T., Saitoh M., Masuoka H., Nakano T., Hidaka H. Selective inhibition of cyclic AMP phosphodiesterase from various human tissues by milrinone, a potent cardiac bipyridine. Biochem Pharmacol. 1988 May 15;37(10):2041–2044. doi: 10.1016/0006-2952(88)90554-0. [DOI] [PubMed] [Google Scholar]
- Kariya T., Dage R. C. Tissue distribution and selective inhibition of subtypes of high affinity cAMP phosphodiesterase. Biochem Pharmacol. 1988 Sep 1;37(17):3267–3270. doi: 10.1016/0006-2952(88)90637-5. [DOI] [PubMed] [Google Scholar]
- Kauffman R. F., Schenck K. W., Utterback B. G., Crowe V. G., Cohen M. L. In vitro vascular relaxation by new inotropic agents: relationship to phosphodiesterase inhibition and cyclic nucleotides. J Pharmacol Exp Ther. 1987 Sep;242(3):864–872. [PubMed] [Google Scholar]
- Komas N., Le Bec A., Stoclet J. C., Lugnier C. Cardiac cGMP-stimulated cyclic nucleotide phosphodiesterases: effects of cGMP analogues and drugs. Eur J Pharmacol. 1991 Jan 25;206(1):5–13. doi: 10.1016/0922-4106(91)90140-d. [DOI] [PubMed] [Google Scholar]
- Komas N., Lugnier C., Le Bec A., Serradeil-Le Gal C., Barthélémy G., Stoclet J. C. Differential sensitivity to cardiotonic drugs of cyclic AMP phosphodiesterases isolated from canine ventricular and sinoatrial-enriched tissues. J Cardiovasc Pharmacol. 1989 Aug;14(2):213–220. doi: 10.1097/00005344-198908000-00005. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Otten A., Frölich J. C., Förstermann U. Endothelial cyclic GMP and cyclic AMP do not regulate the release of endothelium-derived relaxing factor/nitric oxide from bovine aortic endothelial cells. J Pharmacol Exp Ther. 1991 Feb;256(2):677–682. [PubMed] [Google Scholar]
- Lincoln T. M., Thompson M., Cornwell T. L. Purification and characterization of two forms of cyclic GMP-dependent protein kinase from bovine aorta. J Biol Chem. 1988 Nov 25;263(33):17632–17637. [PubMed] [Google Scholar]
- Lugnier C., Schini V. B. Characterization of cyclic nucleotide phosphodiesterases from cultured bovine aortic endothelial cells. Biochem Pharmacol. 1990 Jan 1;39(1):75–84. doi: 10.1016/0006-2952(90)90650-a. [DOI] [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Mülsch A., Busse R. cAMP attenuates autacoid release from endothelial cells: relation to internal calcium. Am J Physiol. 1990 Apr;258(4 Pt 2):H960–H966. doi: 10.1152/ajpheart.1990.258.4.H960. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Phosphodiesterase inhibitors induce endothelium-dependent relaxation of rat and rabbit aorta by potentiating the effects of spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):539–547. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Maurice D. H., Crankshaw D., Haslam R. J. Synergistic actions of nitrovasodilators and isoprenaline on rat aortic smooth muscle. Eur J Pharmacol. 1991 Jan 10;192(2):235–242. doi: 10.1016/0014-2999(91)90048-u. [DOI] [PubMed] [Google Scholar]
- Maurice D. H., Haslam R. J. Nitroprusside enhances isoprenaline-induced increases in cAMP in rat aortic smooth muscle. Eur J Pharmacol. 1990 Dec 4;191(3):471–475. doi: 10.1016/0014-2999(90)94182-w. [DOI] [PubMed] [Google Scholar]
- Muller B., Lugnier C., Stoclet J. C. Implication of cyclic AMP in the positive inotropic effects of cyclic GMP-inhibited cyclic AMP phosphodiesterase inhibitors on guinea pig isolated left atria. J Cardiovasc Pharmacol. 1990 Mar;15(3):444–451. doi: 10.1097/00005344-199003000-00015. [DOI] [PubMed] [Google Scholar]
- Muller B., Lugnier C., Stoclet J. C. Involvement of rolipram-sensitive cyclic AMP phosphodiesterase in the regulation of cardiac contraction. J Cardiovasc Pharmacol. 1990 Nov;16(5):796–803. doi: 10.1097/00005344-199011000-00016. [DOI] [PubMed] [Google Scholar]
- Murali S., Uretsky B. F., Valdes A. M., Kolesar J. A., Reddy P. S. Acute hemodynamic and hormonal effects of CI-930, a new phosphodiesterase inhibitor, in severe congestive heart failure. Am J Cardiol. 1987 Jun 1;59(15):1356–1360. doi: 10.1016/0002-9149(87)90919-2. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. L., Leigh B. K., England P. J. The identification of a new cyclic nucleotide phosphodiesterase activity in human and guinea-pig cardiac ventricle. Implications for the mechanism of action of selective phosphodiesterase inhibitors. Biochem J. 1987 Jan 15;241(2):535–541. doi: 10.1042/bj2410535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M., Nicholson C. D. Comparison of cyclic nucleotide phosphodiesterase isoenzymes in rat and rabbit ventricular myocardium: positive inotropic and phosphodiesterase inhibitory effects of Org 30029, milrinone and rolipram. Naunyn Schmiedebergs Arch Pharmacol. 1990 Dec;342(6):698–705. doi: 10.1007/BF00175715. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Kobylarz-Singer D. C., Keiser J., Haleen S. J., Major T. C., Rapundalo S., Peterson J. T., Panek R. Subclasses of cyclic GMP-specific phosphodiesterase and their role in regulating the effects of atrial natriuretic factor. Hypertension. 1990 May;15(5):528–540. doi: 10.1161/01.hyp.15.5.528. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Kobylarz-Singer D. C., Steffen R. P., Kaplan H. R. Subclasses of cyclic AMP-specific phosphodiesterase in left ventricular muscle and their involvement in regulating myocardial contractility. Circ Res. 1987 Oct;61(4):539–547. doi: 10.1161/01.res.61.4.539. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Baird C. E., Hardman Y. J., Wu J. G. Cyclic nucleotide phosphodiesterase activities of pig coronary arteries. Biochim Biophys Acta. 1975 Apr 19;384(2):430–442. doi: 10.1016/0005-2744(75)90044-3. [DOI] [PubMed] [Google Scholar]



