Abstract
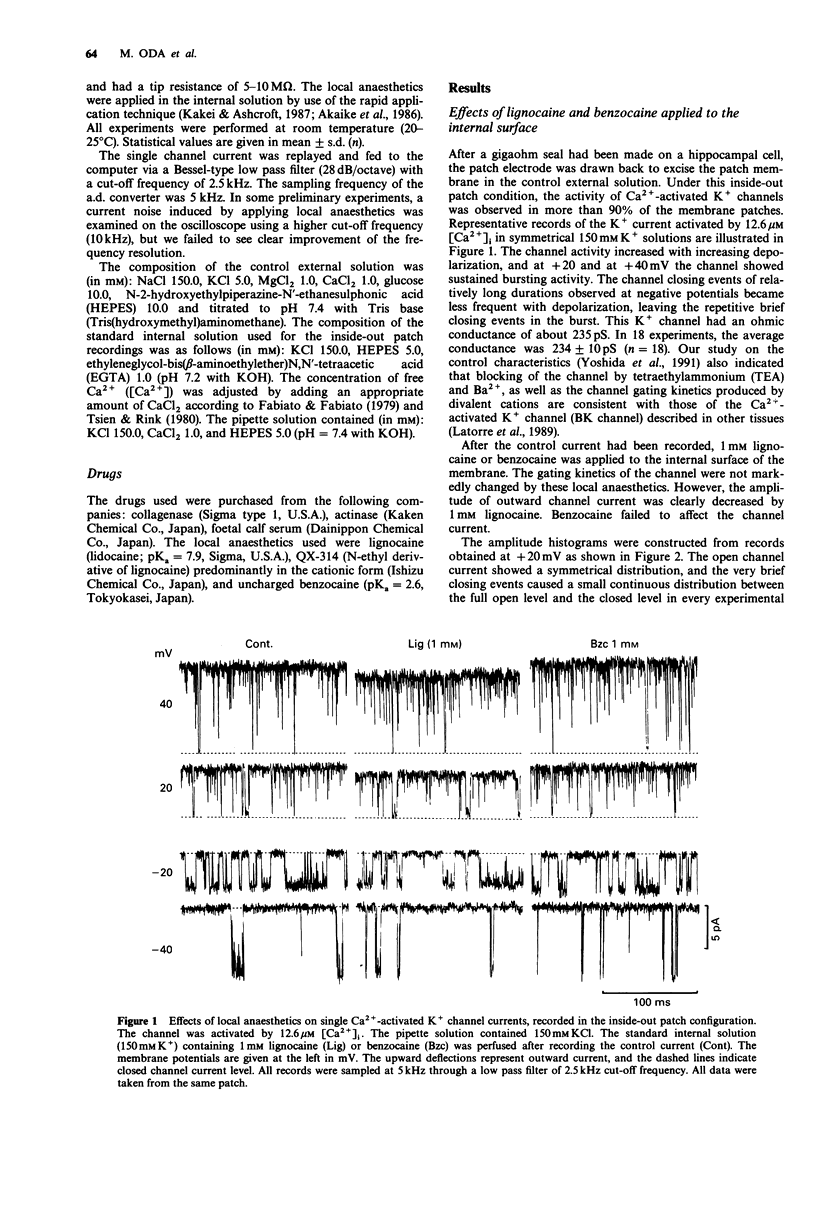
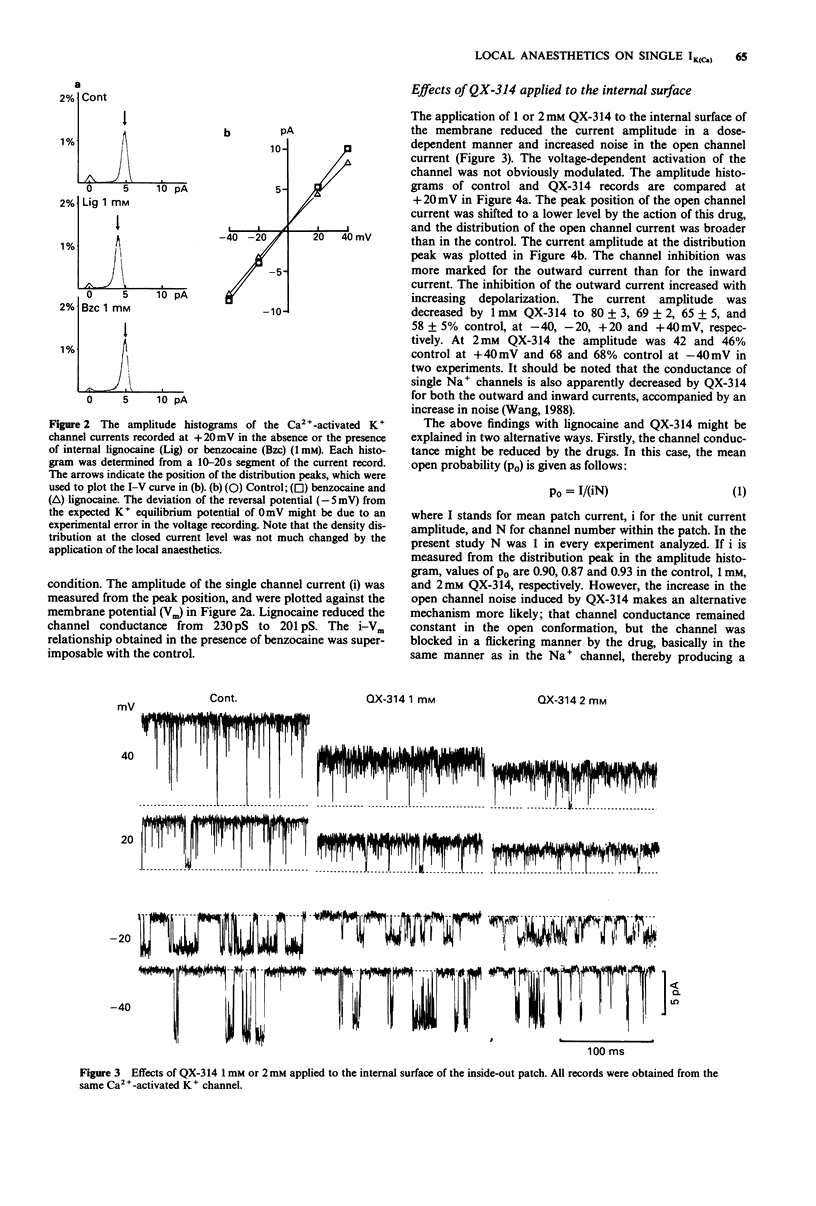
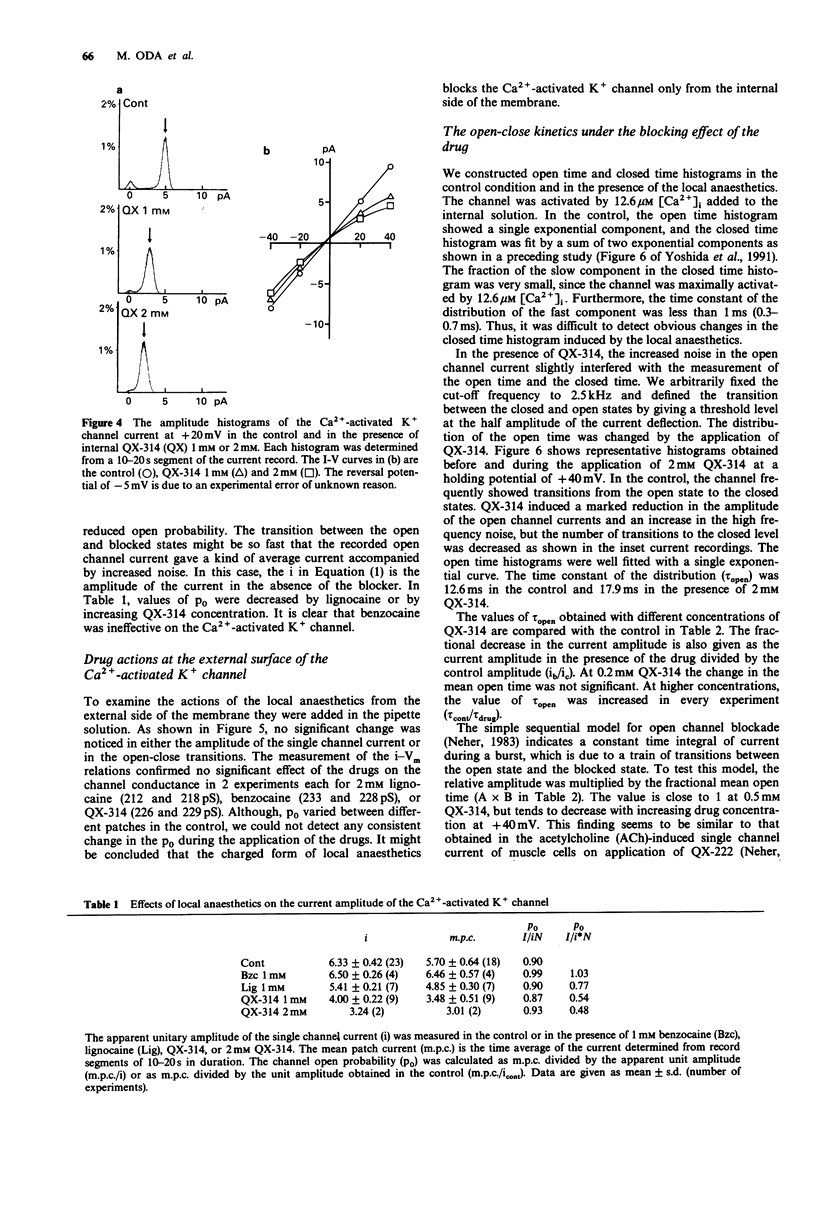
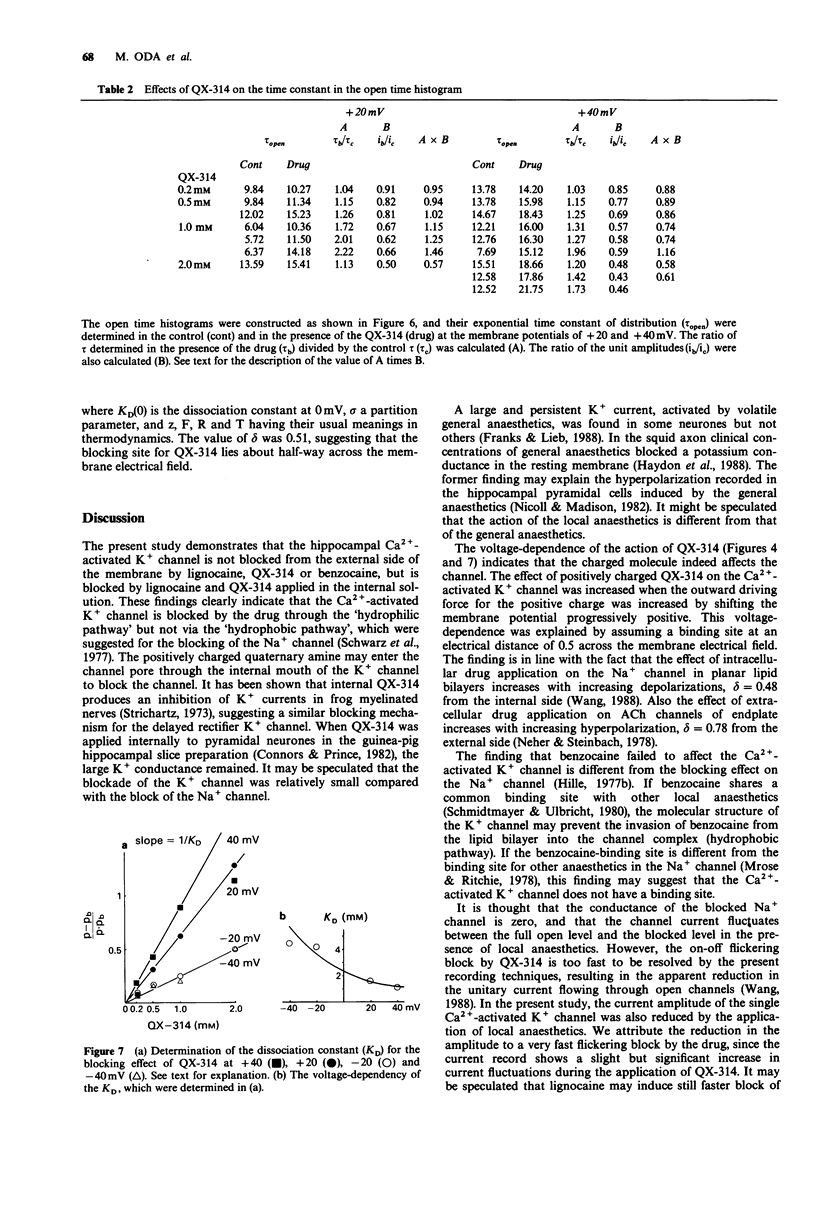
1. Effects of local anaesthetics on single Ca(2+)-activated K+ channels were investigated using the inside-out configuration of the patch-clamp technique in single pyramidal neurones, which were freshly dissociated from rat hippocampus by use of proteolytic enzymes. 2. No significant effect was observed when 2 mM benzocaine was applied on either side of the membrane patch, or when 2 mM lignocaine or QX-314 was applied to the external surface of the membrane. 3. Lignocaine 1 mM, applied to the internal surface, slightly reduced the amplitude of the single K+ channel current. When applied to the internal surface QX-314 reduced the amplitude of the K+ channel current, accompanied by an increase in noise in the open channel current, suggesting a fast flickering block. The blocking effect of QX-314 on the outward current increased with depolarization, suggesting a binding site for the drug at an electrical distance of about 0.5 across the membrane field. 4. The open time histogram showed one exponential component and the closed time histogram showed at least two components. The mean open time of the outward current was increased when the amplitude was reduced by the drugs. 5. The ionized form of the local anaesthetics had a similar action on the Ca(2+)-activated K+ channels to that on Na+ channels, that is, they enter into the channel from the cytoplasmic side to induce open channel block. The blocking kinetics, however, might be so fast that they were beyond the frequency response of our recording apparatus, thus the recorded current amplitude was decreased.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
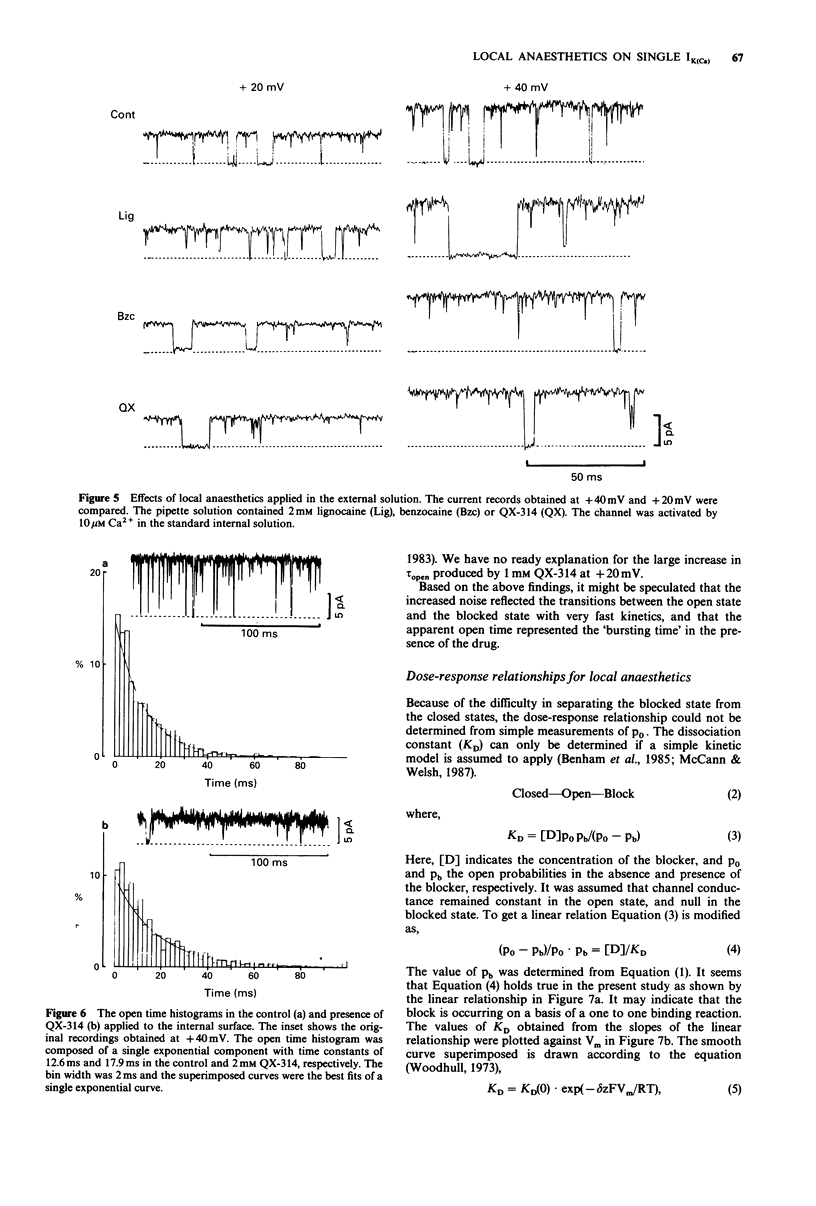


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Prince D. A. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982 Mar;220(3):476–481. [PubMed] [Google Scholar]
- Courtney K. R. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975 Nov;195(2):225–236. [PubMed] [Google Scholar]
- De Jong R. H., Robles R., Corbin R. W. Central actions of lidocaine--synaptic transmission. Anesthesiology. 1969 Jan;30(1):19–23. doi: 10.1097/00000542-196901000-00012. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Volatile general anaesthetics activate a novel neuronal K+ current. Nature. 1988 Jun 16;333(6174):662–664. doi: 10.1038/333662a0. [DOI] [PubMed] [Google Scholar]
- Frazier D. T., Narahashi T., Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):45–51. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Simon A. J. The potassium conductance of the resting squid axon and its blockage by clinical concentrations of general anaesthetics. J Physiol. 1988 Aug;402:363–374. doi: 10.1113/jphysiol.1988.sp017209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol. 1977 Apr;69(4):475–496. doi: 10.1085/jgp.69.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Kakei M., Ashcroft F. M. A microflow superfusion system for use with excised membrane patches. Pflugers Arch. 1987 Jul;409(3):337–341. doi: 10.1007/BF00583487. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Nakamura H., Akaike N. Mechanical and enzymatic isolation of mammalian CNS neurons. Neurosci Res. 1988 Apr;5(4):299–315. doi: 10.1016/0168-0102(88)90032-6. [DOI] [PubMed] [Google Scholar]
- Kuba K., Morita K., Nohmi M. Origin of calcium ions involved in the generation of a slow afterhyperpolarization in bullfrog sympathetic neurones. Pflugers Arch. 1983 Nov;399(3):194–202. doi: 10.1007/BF00656714. [DOI] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Neuroleptics antagonize a calcium-activated potassium channel in airway smooth muscle. J Gen Physiol. 1987 Feb;89(2):339–352. doi: 10.1085/jgp.89.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrose H. E., Ritchie J. M. Local Anesthetics: do benzocaine and lidocaine act at the same single site? J Gen Physiol. 1978 Feb;71(2):223–225. [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Madison D. V. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982 Sep 10;217(4564):1055–1057. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Richards N. W., Dawson D. C. Single potassium channels blocked by lidocaine and quinidine in isolated turtle colon epithelial cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C85–C89. doi: 10.1152/ajpcell.1986.251.1.C85. [DOI] [PubMed] [Google Scholar]
- Schmidtmayer J., Ulbricht W. Interaction of lidocaine and benzocaine in blocking sodium channels. Pflugers Arch. 1980 Aug;387(1):47–54. doi: 10.1007/BF00580843. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Palade P. T., Hille B. Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J. 1977 Dec;20(3):343–368. doi: 10.1016/S0006-3495(77)85554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer C. F. Theoretical characterization of ion channel blockade. Competitive binding to periodically accessible receptors. Biophys J. 1987 Sep;52(3):405–412. doi: 10.1016/S0006-3495(87)83229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Intracellular injection of a Ca2+ chelator inhibits spike repolarization in hippocampal neurons. Brain Res. 1987 Dec 1;435(1-2):387–392. doi: 10.1016/0006-8993(87)91631-3. [DOI] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. Molecular mechanisms of nerve block by local anesthetics. Anesthesiology. 1976 Oct;45(4):421–441. doi: 10.1097/00000542-197610000-00012. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Kuba K. The Ca2+-sensitive K+-currents underlying the slow afterhyperpolarization of bullfrog sympathetic neurones. Pflugers Arch. 1987 Oct;410(3):234–242. doi: 10.1007/BF00580271. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Usubiaga J. E., Wikinski J., Ferrero R., Usubiaga L. E., Wikinski R. Local anesthetic-induced convulsions in man--an electroencephalographic study. Anesth Analg. 1966 Sep-Oct;45(5):611–620. [PubMed] [Google Scholar]
- Wang G. K. Cocaine-induced closures of single batrachotoxin-activated Na+ channels in planar lipid bilayers. J Gen Physiol. 1988 Dec;92(6):747–765. doi: 10.1085/jgp.92.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnick J. E., Kee R. D., Yim G. K. The effects of lidocaine on inhibition in the cerebral cortex. Anesthesiology. 1971 Apr;34(4):327–332. doi: 10.1097/00000542-197104000-00010. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Oda M., Ikemoto Y. Kinetics of the Ca(2+)-activated K+ channel in rat hippocampal neurons. Jpn J Physiol. 1991;41(2):297–315. doi: 10.2170/jjphysiol.41.297. [DOI] [PubMed] [Google Scholar]



