Abstract
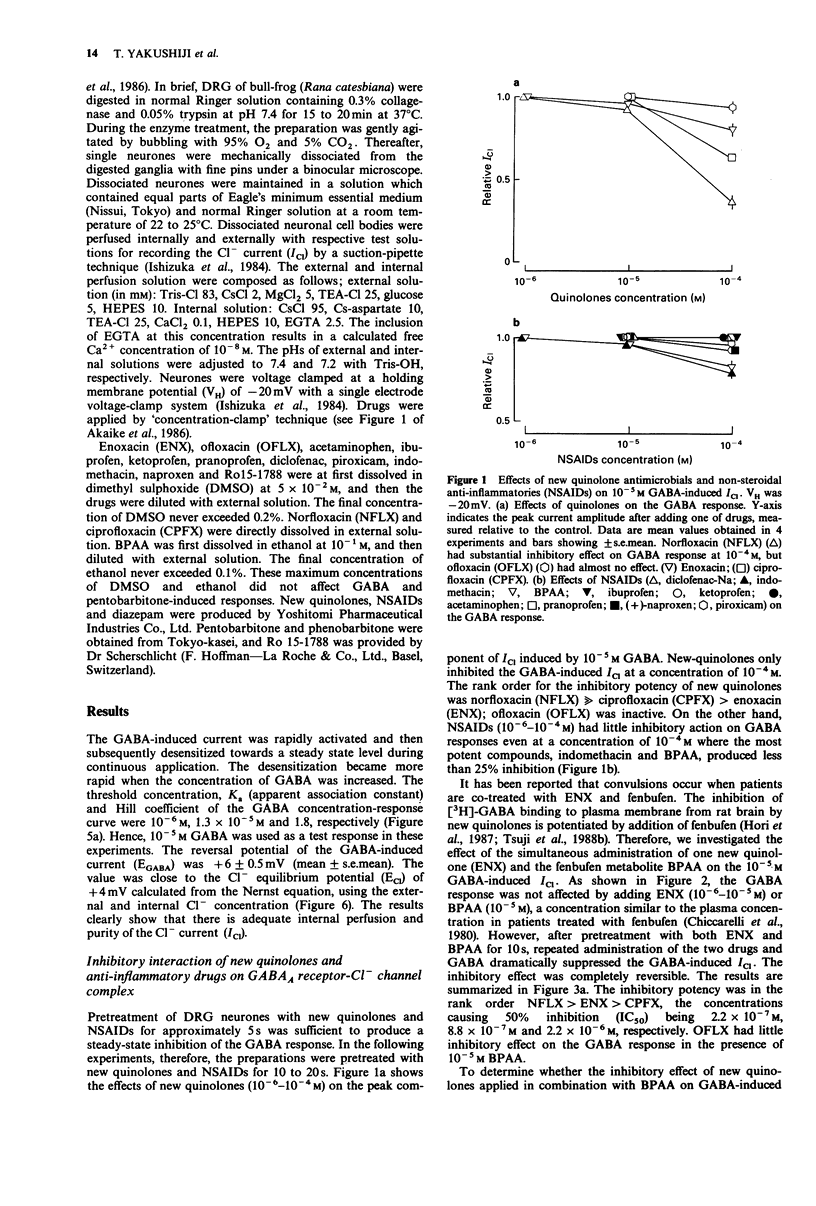
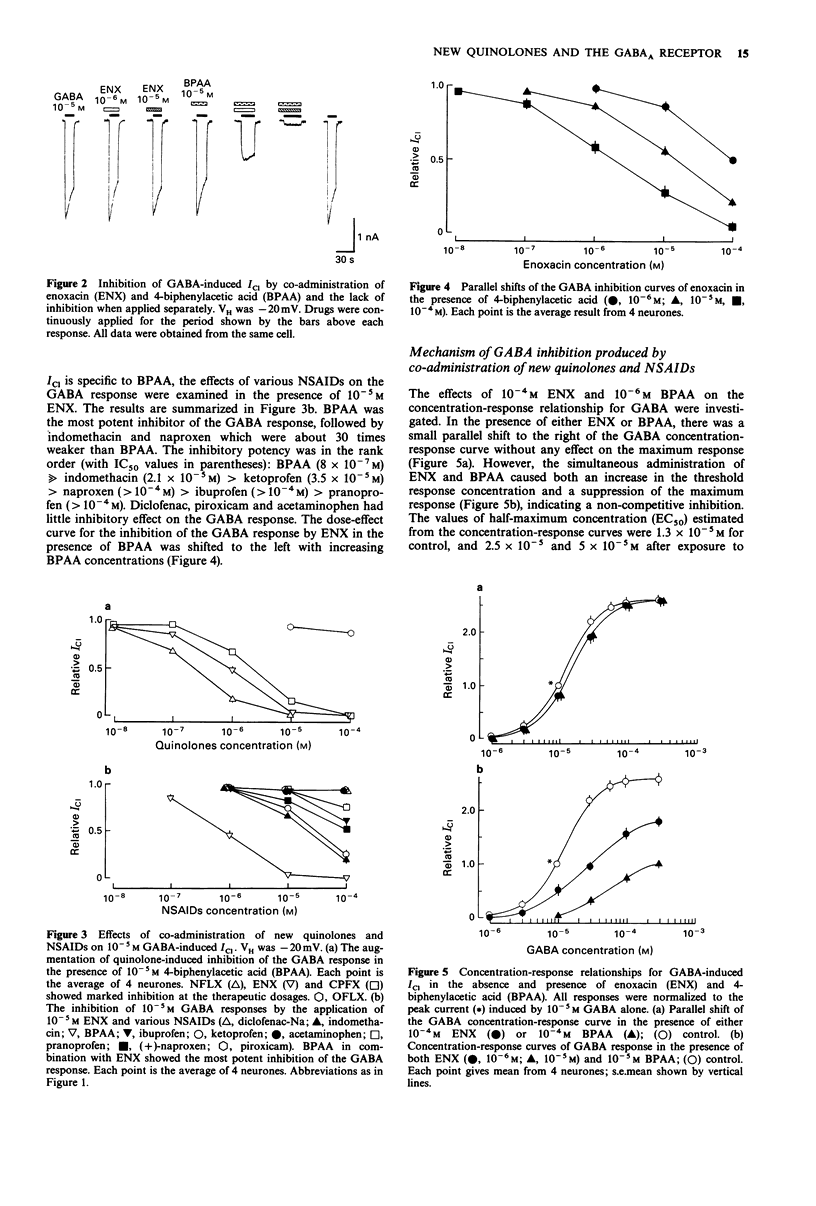
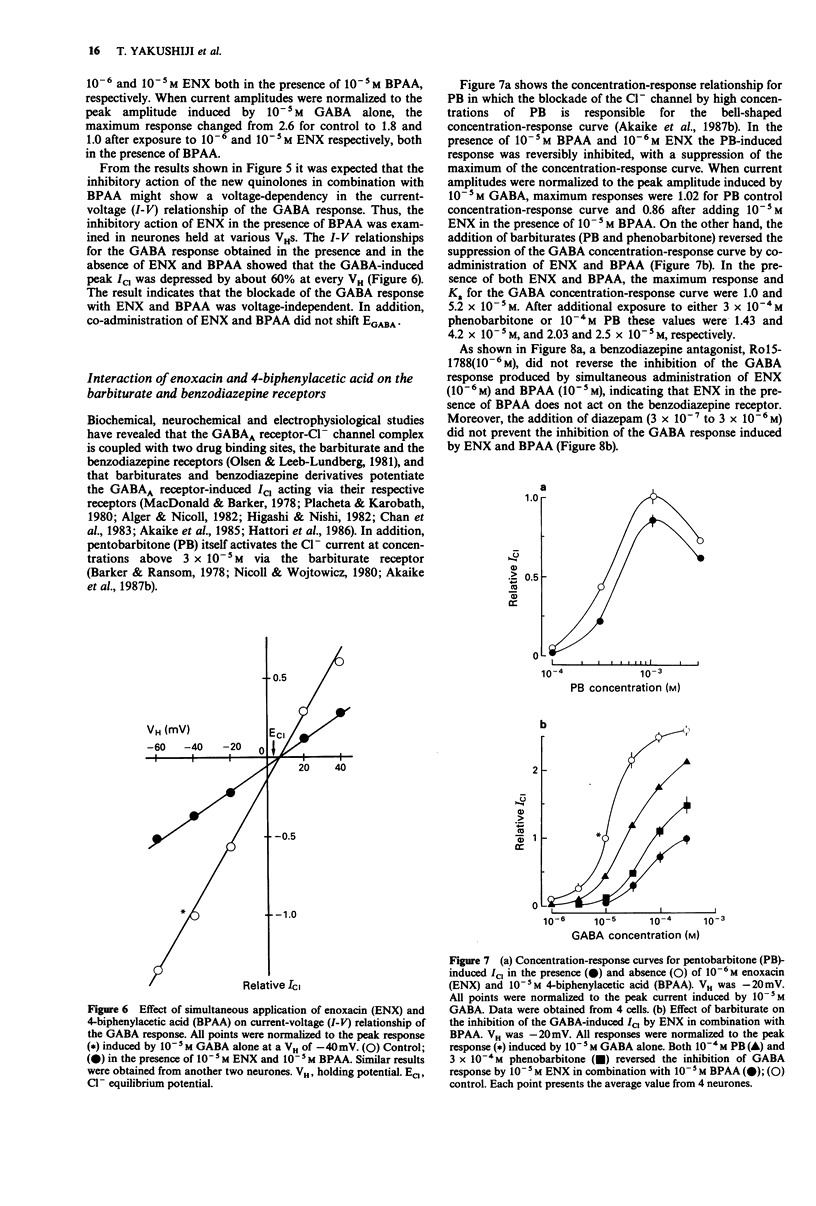
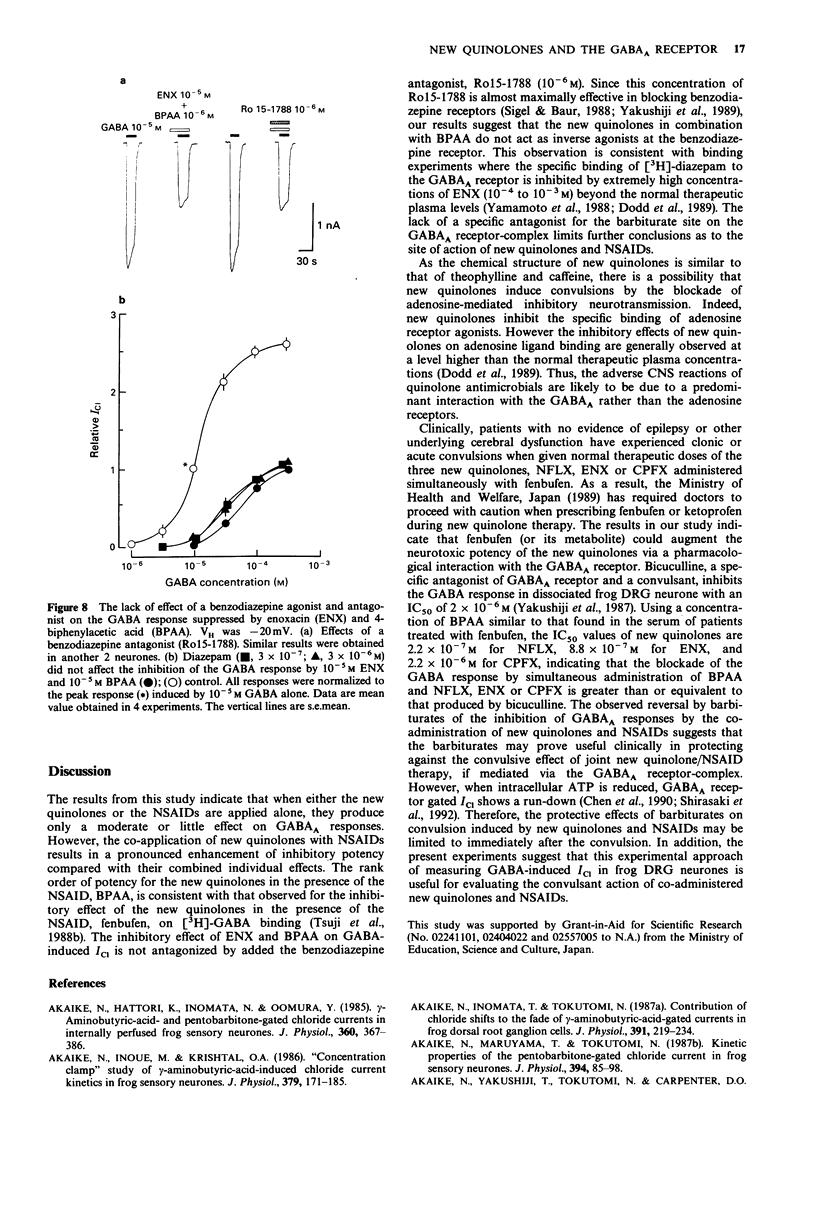
1. The interaction of a new class of quinolone antimicrobials (new quinolones) and non-steroidal anti-inflammatory agents (NSAIDs) with the GABAA receptor-Cl- channel complex was investigated in frog sensory neurones by use of the internal perfusion and 'concentration clamp' techniques. 2. The new quinolones and the NSAIDs (both 10(-6)-10(-5) M) had little effect on the GABA-induced chloride current (ICI) when applied separately. At a concentration of 10(-4) M the new quinolones, and to a lesser degree the NSAIDs, produced some suppression of the GABA response. 3. The co-administration of new quinolones and some NSAIDs (10(-6)-10(-14) M) resulted in a marked suppression of the GABA response. The size of this inhibition was dependent on the concentration of either the new quinolone or the NSAID tested. The inhibitory potency of new quinolones in combination with 4-biphenylacetic acid (BPAA) was in rank order norfloxacin (NFLX) much greater than enoxacin (ENX) greater than ciprofloxancin (CPFX) much greater than ofloxacin (OFLX), and that of NSAIDs in combination with ENX was BPAA much greater than indomethacin = ketoprofen greater than naproxen greater than ibuprofen greater than pranoprofen. Diclofenac, piroxicam and acetaminophen did not affect GABA responses in the presence of ENX. 4. In the presence of ENX or BPAA, there was a small shift to the right of the concentration-response curve for GABA without any effect on the maximum response. However, the co-administration of these drugs suppressed the maximum of the GABA concentration-response curve, indicating a non-competitive inhibition, for which no voltage-dependency was observed.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Inomata N., Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985 Mar;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Inomata N., Tokutomi N. Contribution of chloride shifts to the fade of gamma-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J Physiol. 1987 Oct;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Maruyama T., Tokutomi N. Kinetic properties of the pentobarbitone-gated chloride current in frog sensory neurones. J Physiol. 1987 Dec;394:85–98. doi: 10.1113/jphysiol.1987.sp016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Yakushiji T., Tokutomi N., Carpenter D. O. Multiple mechanisms of antagonism of gamma-aminobutyric acid (GABA) responses. Cell Mol Neurobiol. 1987 Mar;7(1):97–103. doi: 10.1007/BF00734993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P. Adverse reactions and interactions of fluoroquinolones. Clin Invest Med. 1989 Feb;12(1):28–34. [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M. G. The pharmacokinetics and tissue penetration of the fluoroquinolones. Clin Invest Med. 1989 Feb;12(1):20–27. [PubMed] [Google Scholar]
- Chan C. Y., Gibbs T. T., Borden L. A., Farb D. H. Multiple embryonic benzodiazepine binding sites: evidence for functionality. Life Sci. 1983 Nov 21;33(21):2061–2069. doi: 10.1016/0024-3205(83)90329-6. [DOI] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiccarelli F. S., Eisner H. J., Van Lear G. E. Metabolic and pharmacokinetic studies with fenbufen in man. Arzneimittelforschung. 1980;30(4A):728–735. [PubMed] [Google Scholar]
- Dodd P. R., Davies L. P., Watson W. E., Nielsen B., Dyer J. A., Wong L. S., Johnston G. A. Neurochemical studies on quinolone antibiotics: effects on glutamate, GABA and adenosine systems in mammalian CNS. Pharmacol Toxicol. 1989 May;64(5):404–411. doi: 10.1111/j.1600-0773.1989.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Halliwell R. F., Davey P. G., Lambert J. J. The effects of quinolones and NSAIDs upon GABA-evoked currents recorded from rat dorsal root ganglion neurones. J Antimicrob Chemother. 1991 Feb;27(2):209–218. doi: 10.1093/jac/27.2.209. [DOI] [PubMed] [Google Scholar]
- Hattori K., Akaike N., Oomura Y., Kuraoka S. Internal perfusion studies demonstrating GABA-induced chloride responses in frog primary afferent neurons. Am J Physiol. 1984 Mar;246(3 Pt 1):C259–C265. doi: 10.1152/ajpcell.1984.246.3.C259. [DOI] [PubMed] [Google Scholar]
- Hattori K., Oomura Y., Akaike N. Diazepam action on gamma-aminobutyric acid-activated chloride currents in internally perfused frog sensory neurons. Cell Mol Neurobiol. 1986 Sep;6(3):307–323. doi: 10.1007/BF00711116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H., Nishi S. Effect of barbiturates on the GABA receptor of cat primary afferent neurones. J Physiol. 1982 Nov;332:299–314. doi: 10.1113/jphysiol.1982.sp014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Ishizuka S., Hattori K., Akaike N. Separation of ionic currents in the somatic membrane of frog sensory neurons. J Membr Biol. 1984;78(1):19–28. doi: 10.1007/BF01872528. [DOI] [PubMed] [Google Scholar]
- Macdonald R., Barker J. L. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurones. Nature. 1978 Feb 9;271(5645):563–564. doi: 10.1038/271563a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Wojtowicz J. M. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res. 1980 Jun 2;191(1):225–237. doi: 10.1016/0006-8993(80)90325-x. [DOI] [PubMed] [Google Scholar]
- Nicolle L. E. Current experience with quinolones in the treatment of urinary tract infection. Clin Invest Med. 1989 Feb;12(1):35–38. [PubMed] [Google Scholar]
- Olsen R. W., Leeb-Lundberg F. Convulsant and anticonvulsant drug binding sites related to GABA-regulated chloride ion channels. Adv Biochem Psychopharmacol. 1981;26:93–102. [PubMed] [Google Scholar]
- Placheta P., Karobath M. In vitro modulation by SQ 20009 and SQ 65396 of GABA receptor binding in rat CNS membranes. Eur J Pharmacol. 1980 Mar 21;62(2-3):225–228. doi: 10.1016/0014-2999(80)90281-2. [DOI] [PubMed] [Google Scholar]
- Segev S., Rehavi M., Rubinstein E. Quinolones, theophylline, and diclofenac interactions with the gamma-aminobutyric acid receptor. Antimicrob Agents Chemother. 1988 Nov;32(11):1624–1626. doi: 10.1128/aac.32.11.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E., Baur R. Allosteric modulation by benzodiazepine receptor ligands of the GABAA receptor channel expressed in Xenopus oocytes. J Neurosci. 1988 Jan;8(1):289–295. doi: 10.1523/JNEUROSCI.08-01-00289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma E. Structure--activity relationship of quinolones. Clin Invest Med. 1989 Feb;12(1):7–9. [PubMed] [Google Scholar]
- Tsuji A., Sato H., Kume Y., Tamai I., Okezaki E., Nagata O., Kato H. Inhibitory effects of quinolone antibacterial agents on gamma-aminobutyric acid binding to receptor sites in rat brain membranes. Antimicrob Agents Chemother. 1988 Feb;32(2):190–194. doi: 10.1128/aac.32.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Sato H., Okezaki E., Nagata O., Kato H. Effect of the anti-inflammatory agent fenbufen on the quinolone-induced inhibition of gamma-aminobutyric acid binding to rat brain membranes in vitro. Biochem Pharmacol. 1988 Nov 15;37(22):4408–4411. doi: 10.1016/0006-2952(88)90625-9. [DOI] [PubMed] [Google Scholar]
- Vellend H. Role of fluoroquinolones in lower respiratory tract infections. Clin Invest Med. 1989 Feb;12(1):39–43. [PubMed] [Google Scholar]
- Yakushiji T., Fukuda T., Oyama Y., Akaike N. Effects of benzodiazepines and non-benzodiazepine compounds on the GABA-induced response in frog isolated sensory neurones. Br J Pharmacol. 1989 Nov;98(3):735–740. doi: 10.1111/j.1476-5381.1989.tb14600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushiji T., Tokutomi N., Akaike N., Carpenter D. O. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987 Sep;22(3):1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]


