Abstract
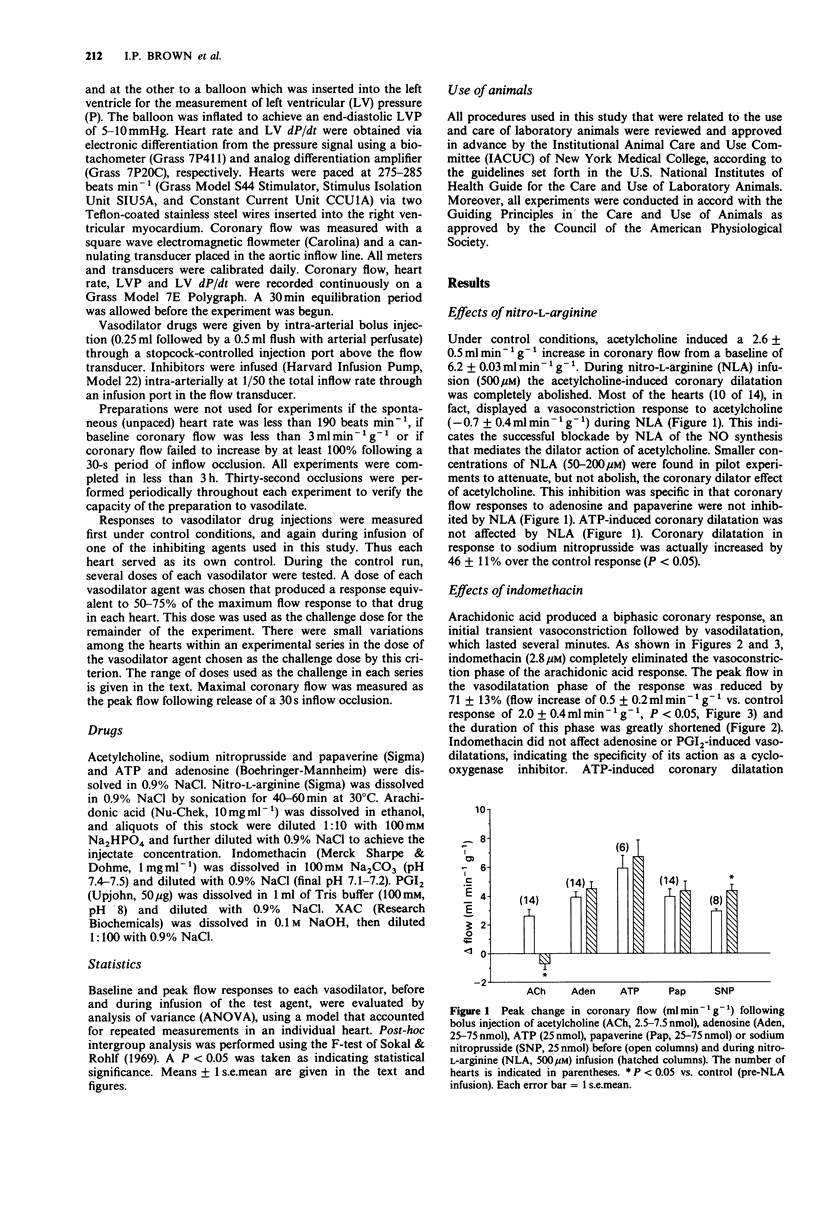
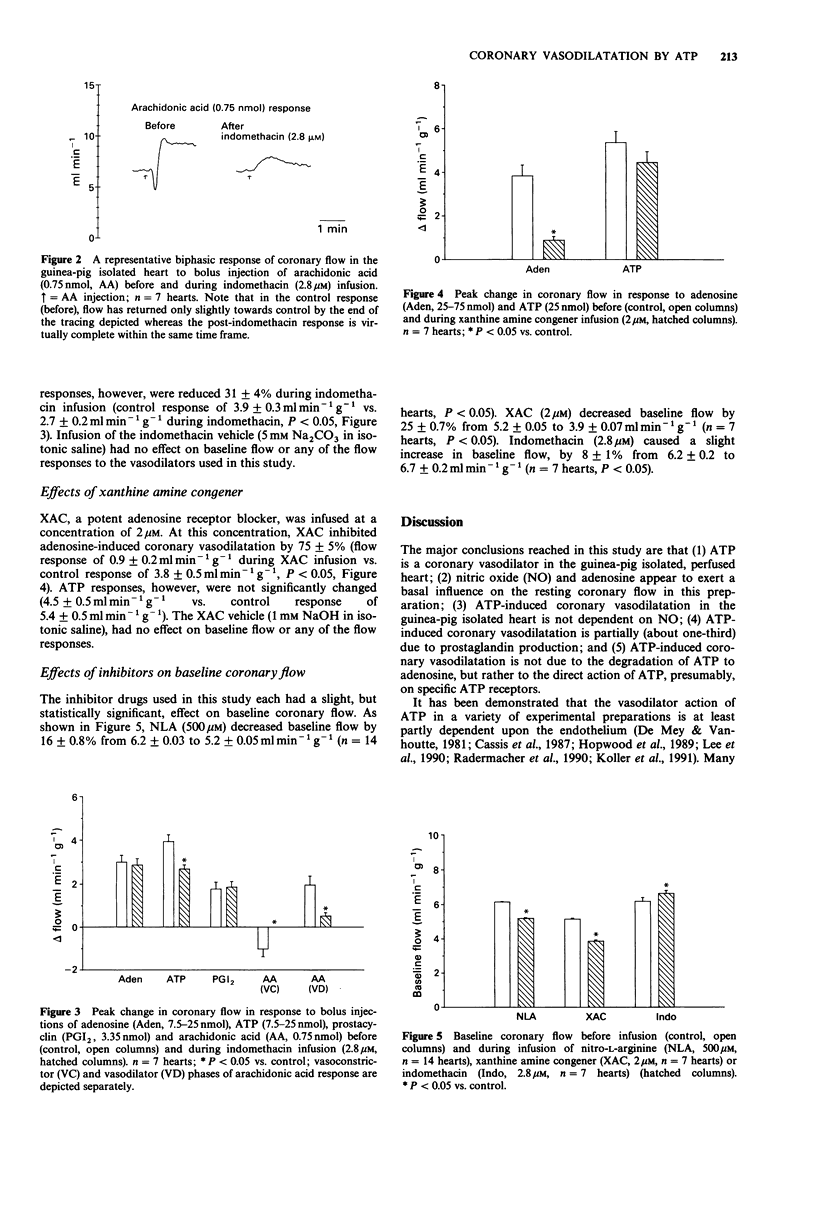
1. Isolated hearts of guinea-pigs were perfused in vitro with a physiological salt solution via a retrograde aortic cannulation (Langendorff preparation) at constant perfusion pressure. Bolus intra-arterial injections of various vasodilator drugs were made and the coronary flow responses were measured with an electromagnetic flow probe placed in the arterial inflow circuit. Inhibitory drugs were infused intra-arterially. 2. Nitro-L-arginine (NLA; 500 microM), an NO synthesis inhibitor, decreased coronary baseline flow by 16 +/- 0.8%, converted acetylcholine-induced coronary vasodilatation to vasoconstriction and had no effect on coronary flow responses to adenosine or papaverine. Sodium nitroprusside-induced responses were enhanced during NLA infusion by 46 +/- 11%. 3. Adenosine 5'-triphosphate (ATP) increased coronary flow but coronary flow responses to ATP were not altered by infusion of NLA. 4. ATP-induced coronary dilatation was not significantly attenuated by infusion of the adenosine receptor antagonist XAC, (xanthine amine congener; 2 microM), whereas XAC decreased coronary flow responses to adenosine by 75% +/- 5%. 5. ATP-induced coronary flow responses were reduced by only 31 +/- 4% during indomethacin infusion (2.8 microM) whereas indomethacin completely eliminated the initial vasoconstriction phase and greatly attenuated the peak flow and duration of the later vasodilatation phase seen in response to arachidonic acid (0.75 nmol). Indomethacin had no effect on vasodilatations produced by adenosine or prostaglandin I2. 6. These results indicate that ATP-induced coronary dilatation in the isolated, perfused heart of the guinea-pig is not dependent upon NO production or upon degradation of ATP to adenosine. The coronary dilator action of ATP may be partially dependent (approximately 30%) upon the production of vasodilator prostaglandins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amezcua J. L., Palmer R. M., de Souza B. M., Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989 Aug;97(4):1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Pohl U., Mülsch A., Bassenge E. Modulation of the vasodilator action of SIN-1 by the endothelium. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S81–S85. doi: 10.1097/00005344-198906152-00015. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol. 1981 Jul;316:347–355. doi: 10.1113/jphysiol.1981.sp013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dézsi L., Simon B., Pohl U., Busse R., Bassenge E. Regulation of femoral vascular resistance by adenine nucleotides via endothelial and smooth muscle receptors. Acta Physiol Hung. 1990;75(1):21–27. [PubMed] [Google Scholar]
- Harlan D. M., Rooke T. W., Belloni F. L., Sparks H. V. Effect of indomethacin on coronary vascular response to increased myocardial oxygen consumption. Am J Physiol. 1978 Oct;235(4):H372–H378. doi: 10.1152/ajpheart.1978.235.4.H372. [DOI] [PubMed] [Google Scholar]
- Hintze T. H., Kaley G. Prostaglandins and the control of blood flow in the canine myocardium. Circ Res. 1977 Mar;40(3):313–320. doi: 10.1161/01.res.40.3.313. [DOI] [PubMed] [Google Scholar]
- Hopwood A. M., Lincoln J., Kirkpatrick K. A., Burnstock G. Adenosine 5'-triphosphate, adenosine and endothelium-derived relaxing factor in hypoxic vasodilatation of the heart. Eur J Pharmacol. 1989 Jun 20;165(2-3):323–326. doi: 10.1016/0014-2999(89)90730-9. [DOI] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Koller A., Rodenburg J. M., Wolin M. S., Messina E. J., Kaley G. Modified arteriolar responses to ATP after impairment of endothelium by light-dye techniques in vivo. Microvasc Res. 1991 Jan;41(1):63–72. doi: 10.1016/0026-2862(91)90008-y. [DOI] [PubMed] [Google Scholar]
- Kroll K., Feigl E. O. Adenosine is unimportant in controlling coronary blood flow in unstressed dog hearts. Am J Physiol. 1985 Dec;249(6 Pt 2):H1176–H1187. doi: 10.1152/ajpheart.1985.249.6.H1176. [DOI] [PubMed] [Google Scholar]
- Lee L., Bruner C. A., Webb R. C. Prostanoids contribute to endothelium-dependent coronary vasodilation in guinea pigs. Blood Vessels. 1990;27(6):341–351. doi: 10.1159/000158828. [DOI] [PubMed] [Google Scholar]
- Mathie R. T., Ralevic V., Alexander B., Burnstock G. Nitric oxide is the mediator of ATP-induced dilatation of the rabbit hepatic arterial vascular bed. Br J Pharmacol. 1991 Jun;103(2):1602–1606. doi: 10.1111/j.1476-5381.1991.tb09834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkes M. S., Douglas J. R., Jr, Needleman P. Prostaglandin release by the isolated perfused rabbit heart. Prostaglandins. 1973 Apr;3(4):439–445. doi: 10.1016/0090-6980(73)90151-2. [DOI] [PubMed] [Google Scholar]
- Moncada S., Rees D. D., Schulz R., Palmer R. M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C. J., Meghji P., Burnstock G. Stimulation of P1-purinoceptors by ATP depends partly on its conversion to AMP and adenosine and partly on direct action. Eur J Pharmacol. 1984 Jan 13;97(1-2):47–54. doi: 10.1016/0014-2999(84)90511-9. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mügge A., Lopez J. A., Piegors D. J., Breese K. R., Heistad D. D. Acetylcholine-induced vasodilatation in rabbit hindlimb in vivo is not inhibited by analogues of L-arginine. Am J Physiol. 1991 Jan;260(1 Pt 2):H242–H247. doi: 10.1152/ajpheart.1991.260.1.H242. [DOI] [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- Needleman P., Minkes M. S., Douglas J. R., Jr Stimulation of prostaglandin biosynthesis by adenine nucleotides. Profile of prostaglandin release by perfused organs. Circ Res. 1974 Apr;34(4):455–460. doi: 10.1161/01.res.34.4.455. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Gordon J. L. Metabolism of adenine nucleotides by ectoenzymes of vascular endothelial and smooth-muscle cells in culture. Biochem J. 1980 Aug 15;190(2):421–429. doi: 10.1042/bj1900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl U., Dézsi L., Simon B., Busse R. Selective inhibition of endothelium-dependent dilation in resistance-sized vessels in vivo. Am J Physiol. 1987 Aug;253(2 Pt 2):H234–H239. doi: 10.1152/ajpheart.1987.253.2.H234. [DOI] [PubMed] [Google Scholar]
- Pohl U., Ogilvie A., Lamontagne D., Busse R. Potent effects of AP3A and AP4A on coronary resistance and autacoid release of intact rabbit hearts. Am J Physiol. 1991 May;260(5 Pt 2):H1692–H1697. doi: 10.1152/ajpheart.1991.260.5.H1692. [DOI] [PubMed] [Google Scholar]
- Radermacher J., Förstermann U., Frölich J. C. Endothelium-derived relaxing factor influences renal vascular resistance. Am J Physiol. 1990 Jul;259(1 Pt 2):F9–17. doi: 10.1152/ajprenal.1990.259.1.F9. [DOI] [PubMed] [Google Scholar]
- Sata T., Kubota E., Said S. I., Misra H. P. EPR spectroscopic studies of detection of a carbon-centered free radical during acetylcholine-induced and endothelium-dependent relaxation of guinea pig pulmonary artery. Free Radic Res Commun. 1990;9(3-6):213–222. doi: 10.3109/10715769009145679. [DOI] [PubMed] [Google Scholar]
- Sunahara F. A., Talesnik J. Myocardial synthesis of prostaglandin-like substances and coronary reactions to cardiostimulation and to hypoxia. Br J Pharmacol. 1979 Jan;65(1):71–85. doi: 10.1111/j.1476-5381.1979.tb17335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talesnik J. Arachidonic acid induced coronary reactions and their inhibition by docosahexaenoic acid. Can J Physiol Pharmacol. 1986 Jan;64(1):77–84. doi: 10.1139/y86-012. [DOI] [PubMed] [Google Scholar]
- Wei H. M., Kang Y. H., Merrill G. F. Coronary vasodilation during global myocardial hypoxia: effects of adenosine deaminase. Am J Physiol. 1988 May;254(5 Pt 2):H1004–H1009. doi: 10.1152/ajpheart.1988.254.5.H1004. [DOI] [PubMed] [Google Scholar]
- Ye Y. X., Akera T., Ng Y. C. Direct actions of gossypol on cardiac muscle. Eur J Pharmacol. 1987 Apr 7;136(1):55–62. doi: 10.1016/0014-2999(87)90778-3. [DOI] [PubMed] [Google Scholar]



