Abstract
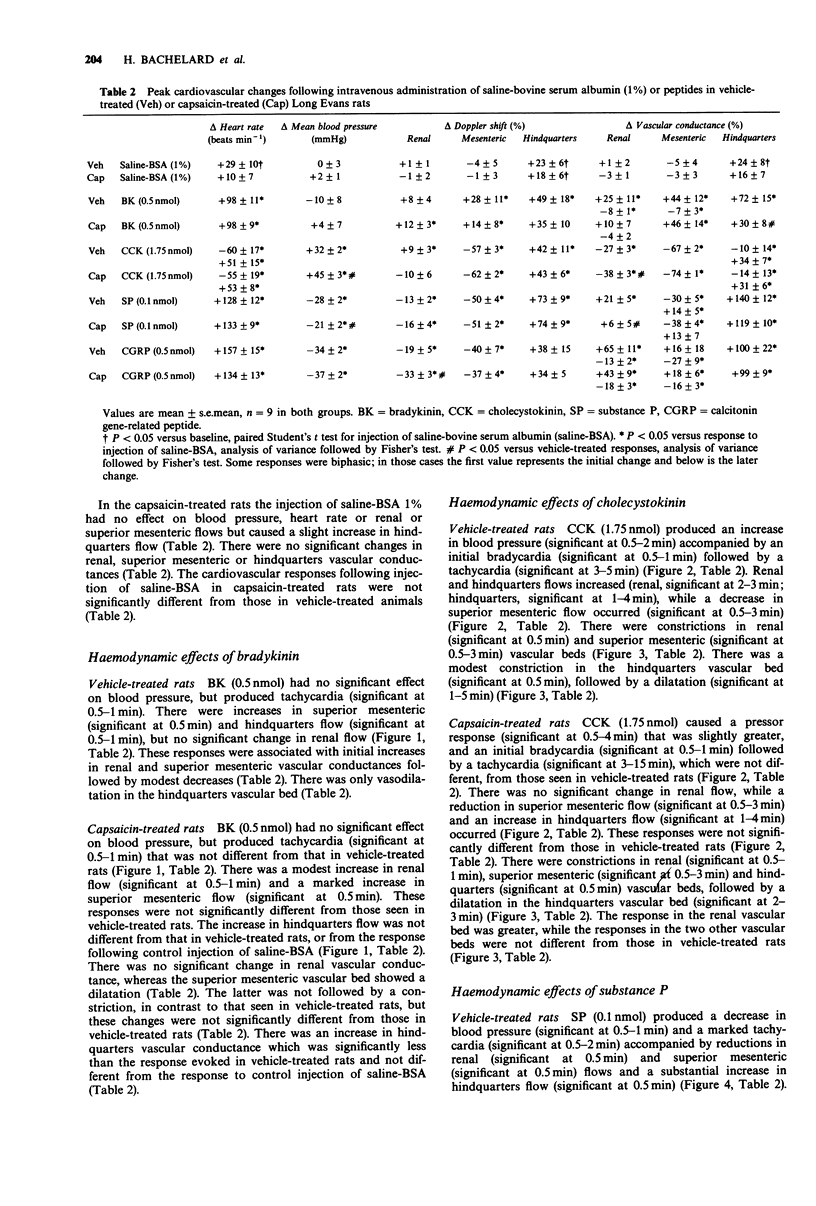
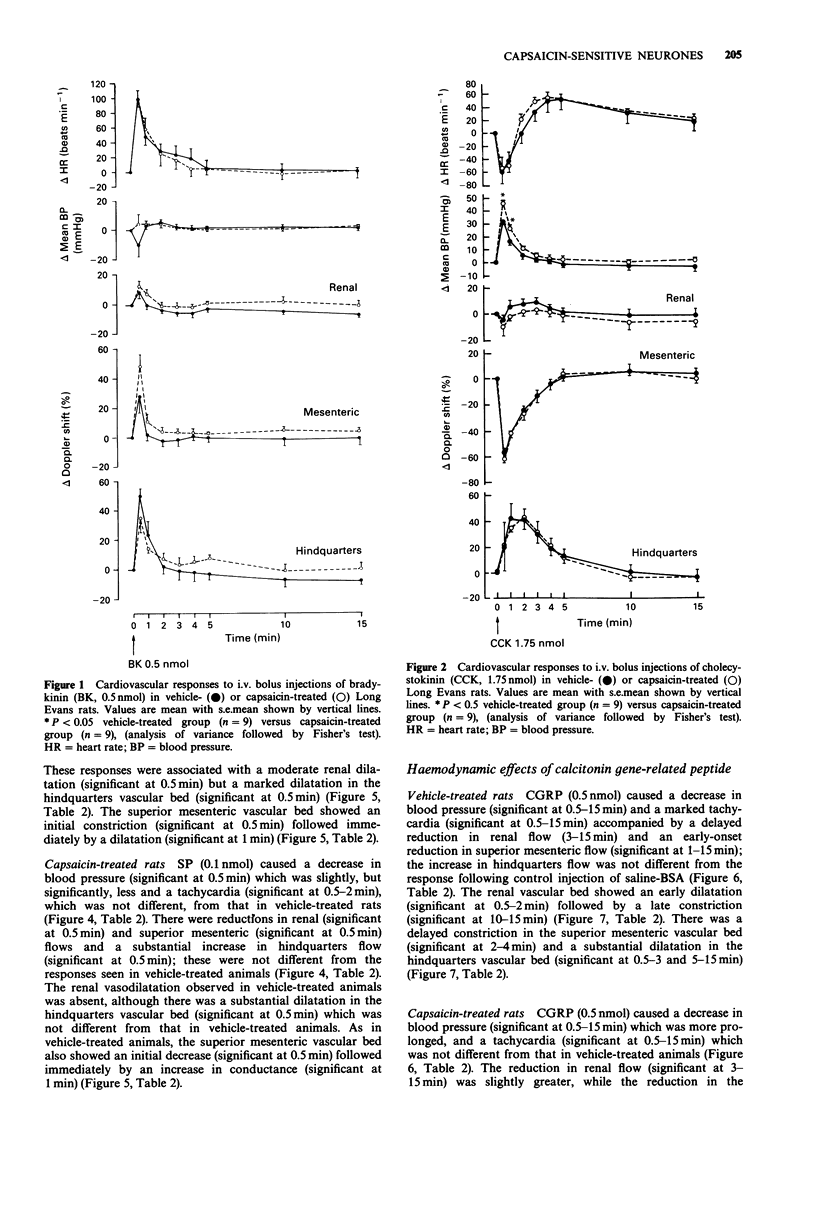
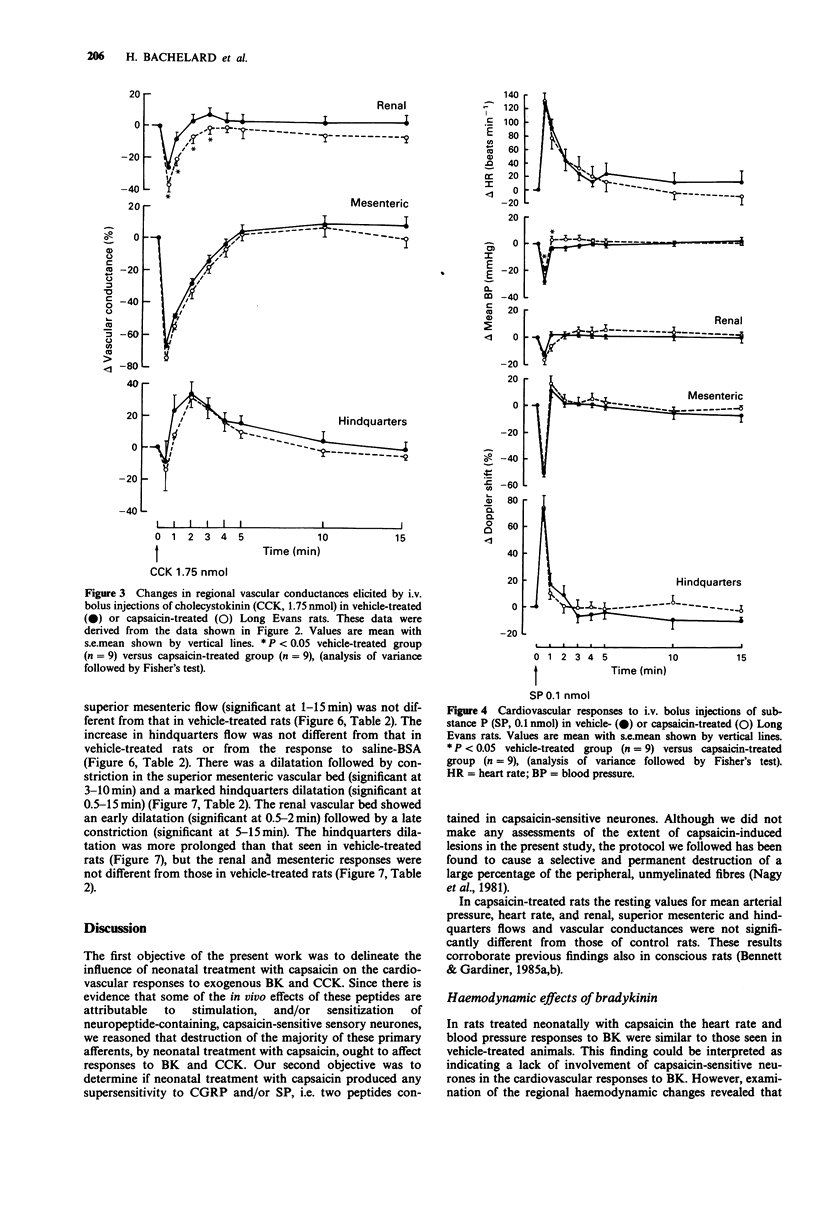
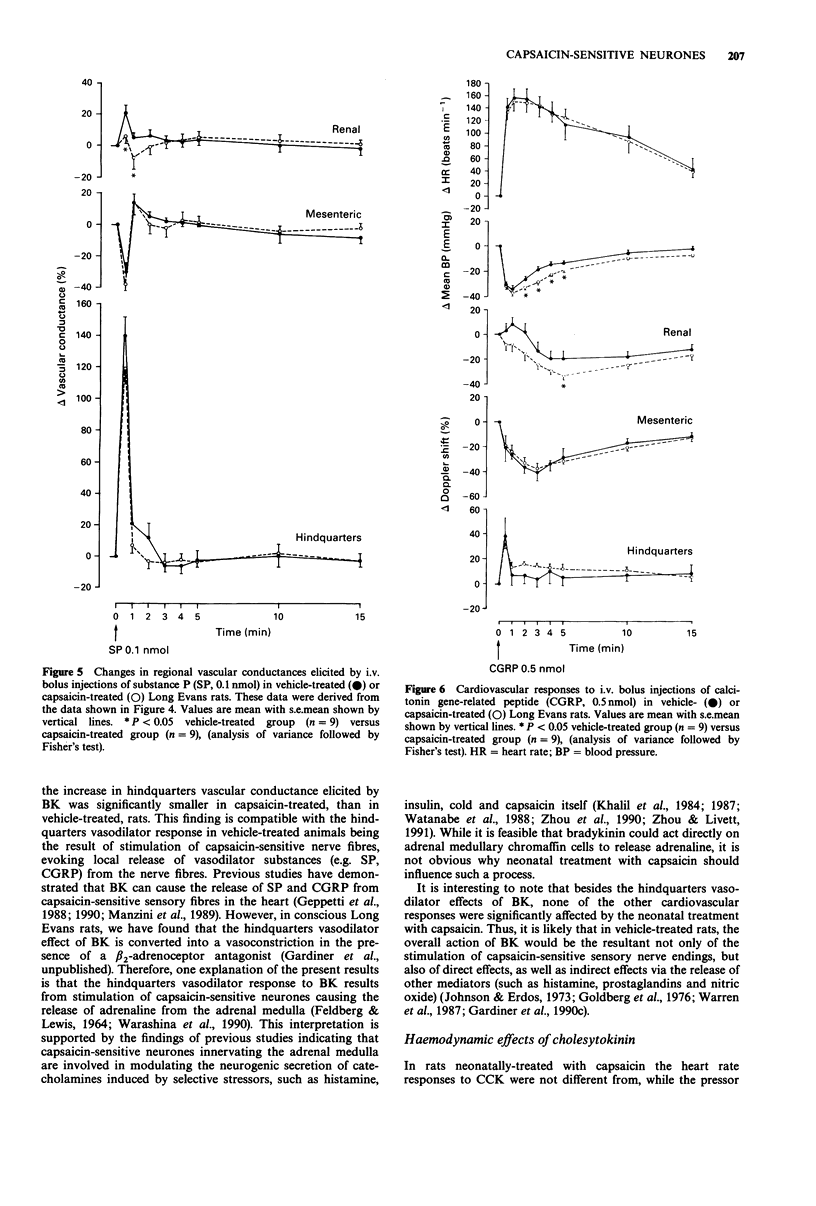
1. The regional haemodynamic effects of i.v. bolus injections of bradykinin (0.05 or 0.5 nmol), cholecystokinin (0.175 or 1.75 nmol), substance P (0.01 or 0.1 nmol) and calcitonin gene-related peptide (0.05 or 0.5 nmol) were assessed in conscious, adult Long Evans rats that had been treated neonatally with either capsaicin (50 mg kg-1, s.c.) or vehicle. 2. In vehicle-treated rats, both doses of bradykinin were without effect on blood pressure, but caused tachycardia and hindquarters vasodilatation. Moreover, after the higher dose there were dilatations in the renal and superior mesenteric vascular beds. In capsaicin-treated rats the hindquarters vasodilator effects elicited by both doses of bradykinin were significantly reduced, while the tachycardia and responses in the renal and superior mesenteric vascular beds were unchanged. 3. In vehicle-treated rats, cholecystokinin caused dose-dependent increases in blood pressure accompanied by renal, superior mesenteric and hindquarters vasoconstriction followed, after the higher dose, by a hindquarters vasodilatation. The lower dose produced a tachycardia, while there was a bradycardia followed by a tachycardia after the higher dose. In capsaicin-treated rats, the pressor response, as well as the renal vasoconstrictor effects of cholecystokinin, were greater than in vehicle-treated rats, while the heart rate, superior mesenteric or hindquarters responses were not different. 4. In vehicle-treated rats, substance P produced a dose-dependent depressor response and tachycardia accompanied by dilatations in the renal and hindquarters vascular beds and constriction in the superior mesenteric vascular bed.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barja F., Mathison R., Huggel H. Substance P-containing nerve fibres in large peripheral blood vessels of the rat. Cell Tissue Res. 1983;229(2):411–422. doi: 10.1007/BF00214982. [DOI] [PubMed] [Google Scholar]
- Bennett T., Gardiner S. M. Pressor sensitivity to exogenous vasopressin in conscious, adult rats treated neonatally with capsaicin. Br J Pharmacol. 1985 May;85(1):115–119. doi: 10.1111/j.1476-5381.1985.tb08837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T., Gardiner S. M. The influence of neonatal treatment with capsaicin on the control of blood pressure in adult rats in water-replete and water-deprived states. Br J Pharmacol. 1985 Aug;85(4):897–903. doi: 10.1111/j.1476-5381.1985.tb11089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Carter D. A., Lightman S. L. Substance P microinjections into the nucleus tractus solitarius elicit a pressor response in capsaicin-treated rats. Neurosci Lett. 1983 Dec 30;43(2-3):253–257. doi: 10.1016/0304-3940(83)90197-0. [DOI] [PubMed] [Google Scholar]
- Cuello A. C., Gamse R., Holzer P., Lembeck F. Substance P immunoreactive neurons following neonatal administration of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jan;315(3):185–194. doi: 10.1007/BF00499834. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., LEWIS G. P. THE ACTION OF PEPTIDES ON THE ADRENAL MEDULLA. RELEASE OF ADRENALINE BY BRADYKININ AND ANGIOTENSIN. J Physiol. 1964 May;171:98–108. doi: 10.1113/jphysiol.1964.sp007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Cereceda A., Henke H., Lundberg J. M., Petermann J. B., Hökfelt T., Fischer J. A. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987 Mar-Apr;8(2):399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Papka R. E., Della N. G., Costa M., Eskay R. L. Substance P-like immunoreactivity in nerves associated with the vascular system of guinea-pigs. Neuroscience. 1982 Feb;7(2):447–459. doi: 10.1016/0306-4522(82)90279-2. [DOI] [PubMed] [Google Scholar]
- Gamse R., Holzer P., Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980 Feb;68(2):207–213. doi: 10.1111/j.1476-5381.1980.tb10409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Hartley C. J. Can pulsed Doppler technique measure changes in aortic blood flow in conscious rats? Am J Physiol. 1990 Aug;259(2 Pt 2):H448–H456. doi: 10.1152/ajpheart.1990.259.2.H448. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T. Regional haemodynamic effects of depressor neuropeptides in conscious, unrestrained, Long Evans and Brattleboro rats. Br J Pharmacol. 1988 Sep;95(1):197–208. doi: 10.1111/j.1476-5381.1988.tb16565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T. Regional haemodynamic effects of human alpha- and beta-calcitonin gene-related peptide in conscious Wistar rats. Br J Pharmacol. 1989 Dec;98(4):1225–1232. doi: 10.1111/j.1476-5381.1989.tb12668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
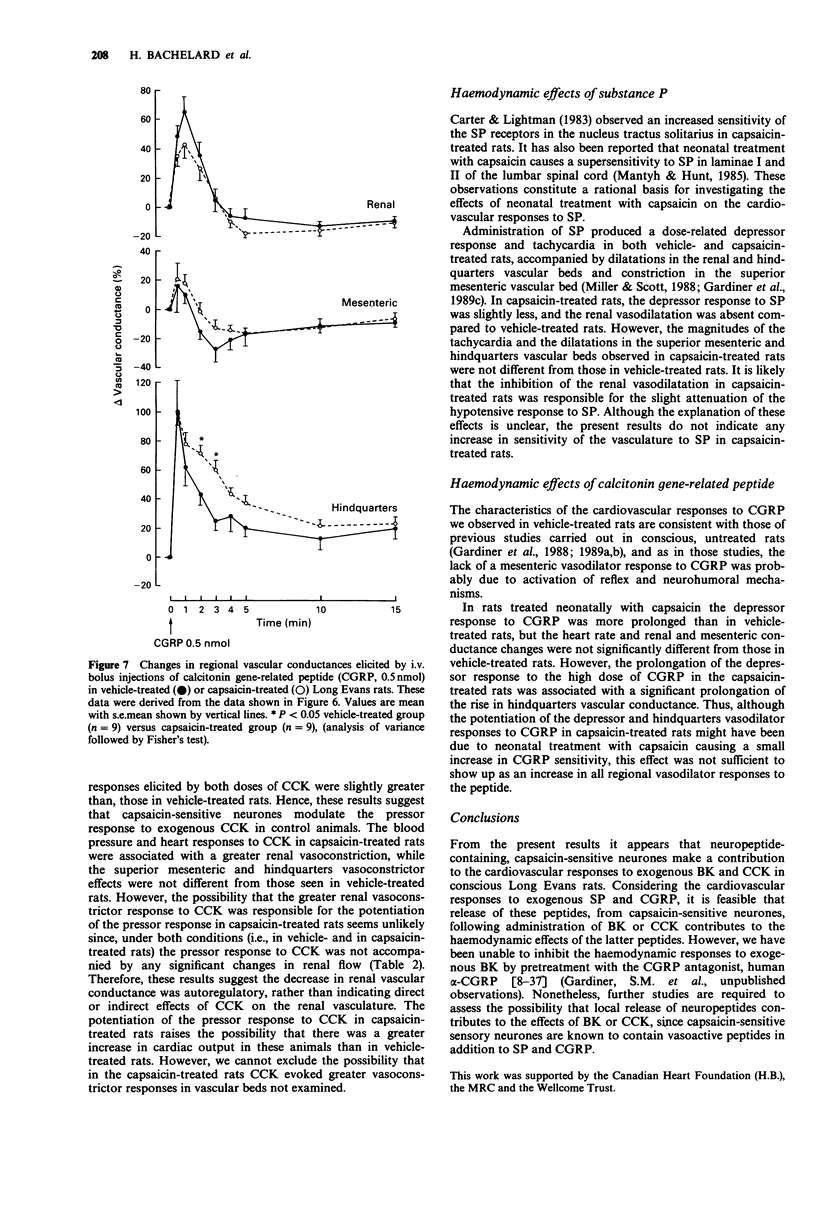
- Gardiner S. M., Compton A. M., Bennett T. Regional hemodynamic effects of calcitonin gene-related peptide. Am J Physiol. 1989 Feb;256(2 Pt 2):R332–R338. doi: 10.1152/ajpregu.1989.256.2.R332. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P., Maggi C. A., Perretti F., Frilli S., Manzini S. Simultaneous release by bradykinin of substance P- and calcitonin gene-related peptide immunoreactivities from capsaicin-sensitive structures in guinea-pig heart. Br J Pharmacol. 1988 Jun;94(2):288–290. doi: 10.1111/j.1476-5381.1988.tb11528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P., Tramontana M., Santicioli P., Del Bianco E., Giuliani S., Maggi C. A. Bradykinin-induced release of calcitonin gene-related peptide from capsaicin-sensitive nerves in guinea-pig atria: mechanism of action and calcium requirements. Neuroscience. 1990;38(3):687–692. doi: 10.1016/0306-4522(90)90062-9. [DOI] [PubMed] [Google Scholar]
- Goldberg M. R., Chapnick B. M., Joiner P. D., Hyman A. L., Kadowitz P. J. Influence of inhibitors of prostaglandin synthesis on venoconstrictor responses to bradykinin. J Pharmacol Exp Ther. 1976 Aug;198(2):357–365. [PubMed] [Google Scholar]
- Haywood J. R., Shaffer R. A., Fastenow C., Fink G. D., Brody M. J. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rat. Am J Physiol. 1981 Aug;241(2):H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- Hottenstein O. D., Pawlik W. W., Remak G., Jacobson E. D. Capsaicin-sensitive nerves modulate resting blood flow and vascular tone in rat gut. Naunyn Schmiedebergs Arch Pharmacol. 1991 Feb;343(2):179–184. doi: 10.1007/BF00168607. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med. 1973 Apr;142(4):1252–1256. doi: 10.3181/00379727-142-37219. [DOI] [PubMed] [Google Scholar]
- Khalil Z., Livett B. G., Marley P. D. Sensory fibres modulate histamine-induced catecholamine secretion from the rat adrenal medulla and sympathetic nerves. J Physiol. 1987 Oct;391:511–526. doi: 10.1113/jphysiol.1987.sp016753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil Z., Marley P. D., Livett B. G. Neonatal capsaicin treatment prevents insulin-stress-induced adrenal catecholamine secretion in vivo: possible involvement of sensory nerves containing substance P. Neurosci Lett. 1984 Mar 9;45(1):65–70. doi: 10.1016/0304-3940(84)90330-6. [DOI] [PubMed] [Google Scholar]
- Lappe R. W., Slivjak M. J., Todt J. A., Wendt R. L. Hemodynamic effects of calcitonin gene-related peptide in conscious rats. Regul Pept. 1987 Dec;19(5-6):307–312. doi: 10.1016/0167-0115(87)90172-8. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Franco-Cereceda A., Hua X., Hökfelt T., Fischer J. A. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol. 1985 Feb 5;108(3):315–319. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W., Hunt S. P. The autoradiographic localization of substance P receptors in the rat and bovine spinal cord and the rat and cat spinal trigeminal nucleus pars caudalis and the effects of neonatal capsaicin. Brain Res. 1985 Apr 22;332(2):315–324. doi: 10.1016/0006-8993(85)90600-6. [DOI] [PubMed] [Google Scholar]
- Manzini S., Perretti F., De Benedetti L., Pradelles P., Maggi C. A., Geppetti P. A comparison of bradykinin- and capsaicin-induced myocardial and coronary effects in isolated perfused heart of guinea-pig: involvement of substance P and calcitonin gene-related peptide release. Br J Pharmacol. 1989 Jun;97(2):303–312. doi: 10.1111/j.1476-5381.1989.tb11955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martling C. R., Saria A., Fischer J. A., Hökfelt T., Lundberg J. M. Calcitonin gene-related peptide and the lung: neuronal coexistence with substance P, release by capsaicin and vasodilatory effect. Regul Pept. 1988 Feb;20(2):125–139. doi: 10.1016/0167-0115(88)90046-8. [DOI] [PubMed] [Google Scholar]
- McCann M. J., Verbalis J. G., Stricker E. M. Capsaicin pretreatment attenuates multiple responses to cholecystokinin in rats. J Auton Nerv Syst. 1988 Sep;23(3):265–272. doi: 10.1016/0165-1838(88)90101-4. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Hunt S. P., Iversen L. L., Emson P. C. Biochemical and anatomical observations on the degeneration of peptide-containing primary afferent neurons after neonatal capsaicin. Neuroscience. 1981;6(10):1923–1934. doi: 10.1016/0306-4522(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Rózsa Z., Jancsó G., Varró V. Possible involvement of capsaicin-sensitive sensory nerves in the regulation of intestinal blood flow in the dog. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jul;326(4):352–356. doi: 10.1007/BF00501442. [DOI] [PubMed] [Google Scholar]
- Sigrist S., Franco-Cereceda A., Muff R., Henke H., Lundberg J. M., Fischer J. A. Specific receptor and cardiovascular effects of calcitonin gene-related peptide. Endocrinology. 1986 Jul;119(1):381–389. doi: 10.1210/endo-119-1-381. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Calcitonin gene-related peptide coexists with substance P in capsaicin sensitive neurons and sensory ganglia of the rat. Peptides. 1985 Jul-Aug;6(4):747–754. doi: 10.1016/0196-9781(85)90179-2. [DOI] [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Ekblad E., Håkanson R., Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. 1986 Aug;15(1):1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Ritter J. M., Hickling N. E., Barrow S. E. Bradykinin-stimulated prostaglandin synthesis in conscious rabbits. Br J Pharmacol. 1987 Dec;92(4):895–900. doi: 10.1111/j.1476-5381.1987.tb11395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Kawada T., Iwai K. Effect of capsaicin pretreatment on capsaicin-induced catecholamine secretion from the adrenal medulla in rats. Proc Soc Exp Biol Med. 1988 Mar;187(3):370–374. doi: 10.3181/00379727-187-3-rc1. [DOI] [PubMed] [Google Scholar]
- Wharton J., Gulbenkian S., Mulderry P. K., Ghatei M. A., McGregor G. P., Bloom S. R., Polak J. M. Capsaicin induces a depletion of calcitonin gene-related peptide (CGRP)-immunoreactive nerves in the cardiovascular system of the guinea pig and rat. J Auton Nerv Syst. 1986 Aug;16(4):289–309. doi: 10.1016/0165-1838(86)90035-4. [DOI] [PubMed] [Google Scholar]
- Wharton J., Gulbenkian S. Peptides in the mammalian cardiovascular system. Experientia. 1987 Jul 15;43(7):821–832. doi: 10.1007/BF01945360. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Angus J. A., Korner P. I. Vascular amplifier properties in renovascular hypertension in conscious rabbits. Hindquarter responses to constrictor and dilator stimuli. Hypertension. 1987 Feb;9(2):122–131. doi: 10.1161/01.hyp.9.2.122. [DOI] [PubMed] [Google Scholar]
- Zhou X. F., Livett B. G. Capsaicin-sensitive sensory neurons are involved in the plasma catecholamine response of rats to selective stressors. J Physiol. 1991 Feb;433:393–407. doi: 10.1113/jphysiol.1991.sp018433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. F., Marley P. D., Livett B. G. Role of capsaicin-sensitive neurons in catecholamine secretion from rat adrenal glands. Eur J Pharmacol. 1990 Sep 21;186(2-3):247–255. doi: 10.1016/0014-2999(90)90440-h. [DOI] [PubMed] [Google Scholar]



