Abstract
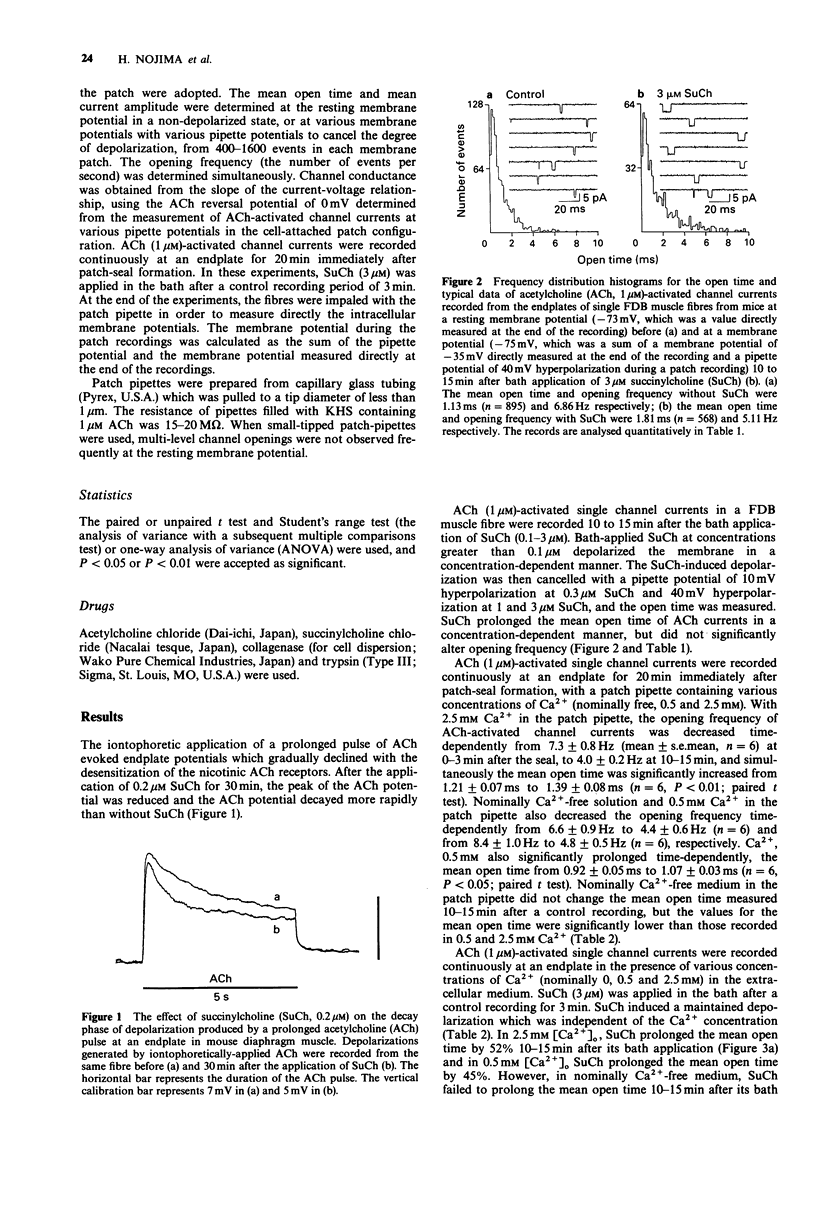
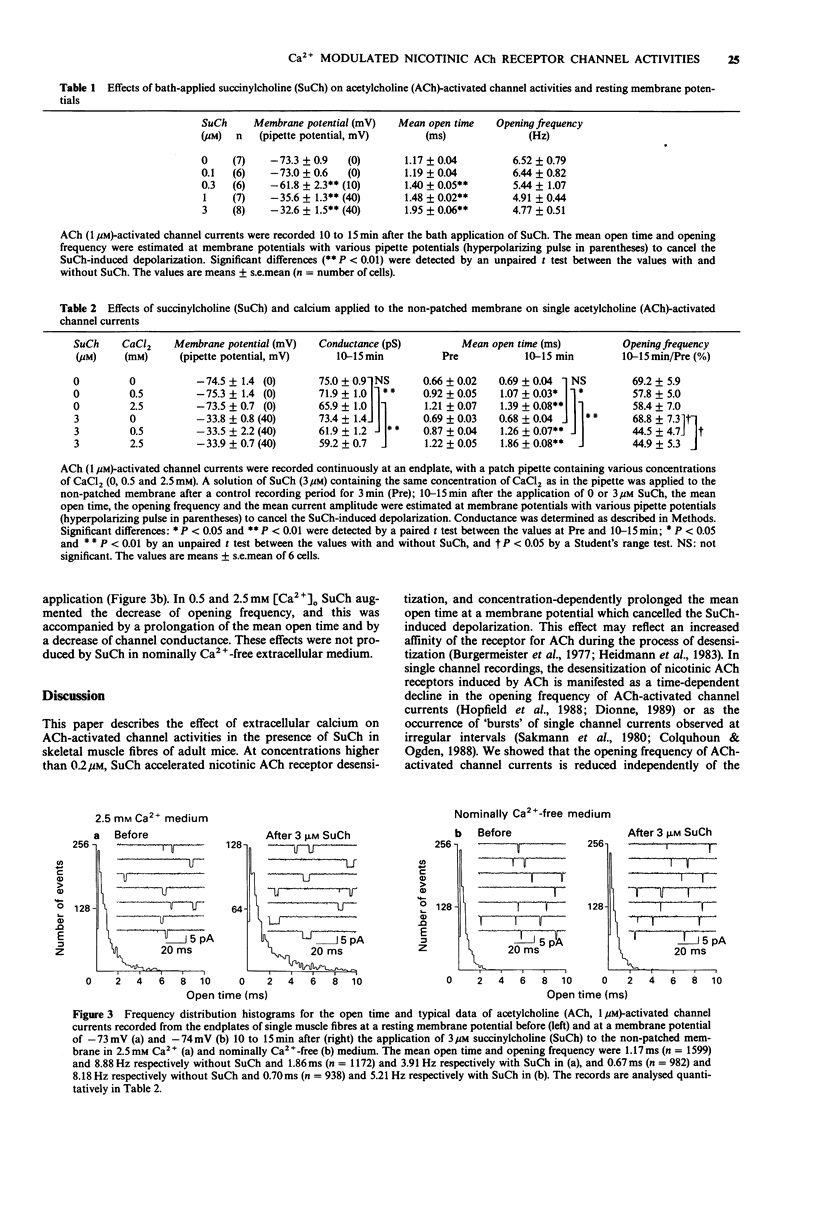
1. The effect of extracellular calcium on single acetylcholine (ACh)-activated channel activities when desensitizing concentrations of succinylcholine (SuCh) were applied to the surrounding endplate membrane was investigated by the cell-attached patch-clamp technique at endplates of single skeletal muscle (flexor digitorum brevis) fibres of adult mice. 2. Bath-applied SuCh (0.1-3 microM, in 2.5 mM Ca2+) increased in a concentration-dependent manner the mean open time of ACh-activated channel currents recorded at membrane potentials which cancelled the SuCh-induced depolarizations. 3. In the presence of 0.5 and 2.5 mM external Ca2+, SuCh (3 microM) applied outside the patch pipette prolonged the mean open time of ACh-activated channel currents in a time-dependent manner (by 45% and 52%, respectively), and simultaneously significantly decreased the single channel conductance (by 14% and 10%, respectively). These SuCh-induced effects did not occur in a nominally Ca(2+)-free extracellular medium. 4. Under the same conditions, SuCh (3 microM) augmented the time-dependent decline in the opening frequency of ACh-activated channel currents obtained in nominally Ca(2+)-free medium. 5. These results suggest that external calcium ions act to modulate nicotinic ACh receptor channel activity, and accelerate desensitization of the receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R., Narahashi T. Comparison of desensitization and time-dependent block of the acetylcholine receptor responses by chlorpromazine, cytochalasin B, triton X-100 and other agents. Br J Pharmacol. 1980 May;69(1):99–106. doi: 10.1111/j.1476-5381.1980.tb10887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracava Y., Ikeda S. R., Daly J. W., Brookes N., Albuquerque E. X. Interactions of bupivacaine with ionic channels of the nicotinic receptor. Analysis of single-channel currents. Mol Pharmacol. 1984 Sep;26(2):304–313. [PubMed] [Google Scholar]
- Burgermeister W., Catterall W. A., Witkop B. Histrionicotoxin enhances agonist-induced desensitization of acetylcholine receptor. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5754–5758. doi: 10.1073/pnas.74.12.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E. Two types of nicotinic acetylcholine receptor channels at slow fibre end-plates of the garter snake. J Physiol. 1989 Feb;409:313–331. doi: 10.1113/jphysiol.1989.sp017499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant N. N., Katz R. L. Suxamethonium. Br J Anaesth. 1982 Feb;54(2):195–208. doi: 10.1093/bja/54.2.195. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Grassi F., Molinaro M., Zani B. M. Acetylcholine regulation of nicotinic receptor channels through a putative G protein in chick myotubes. J Physiol. 1987 Dec;393:635–645. doi: 10.1113/jphysiol.1987.sp016845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiekers J. F., Spannbauer P. M., Scubon-Mulieri B., Parsons R. L. Voltage dependence of desensitization. Influence of calcium and activation kinetics. J Gen Physiol. 1980 May;75(5):511–529. doi: 10.1085/jgp.75.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. S., Nakajima S., Nakajima Y. Functional properties of newly inserted acetylcholine receptors in embryonic Xenopus muscle cells. Brain Res. 1985 Apr;351(2):289–296. doi: 10.1016/0165-3806(85)90200-7. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Oswald R. E., Changeux J. P. Multiple sites of action for noncompetitive blockers on acetylcholine receptor rich membrane fragments from torpedo marmorata. Biochemistry. 1983 Jun 21;22(13):3112–3127. doi: 10.1021/bi00282a014. [DOI] [PubMed] [Google Scholar]
- Hopfield J. F., Tank D. W., Greengard P., Huganir R. L. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988 Dec 15;336(6200):677–680. doi: 10.1038/336677a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I., Kondoh T., Kimura M. Changes in intracellular Ca2+ produced in the mouse diaphragm by neuromuscular blocking drugs. J Pharm Pharmacol. 1990 Sep;42(9):626–631. doi: 10.1111/j.2042-7158.1990.tb06620.x. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Nikolsky E., Vyskocil F. Effect of the desensitization-potentiating agent SKF-525a on frog end-plate currents. Eur J Pharmacol. 1982 May 7;80(1):115–119. doi: 10.1016/0014-2999(82)90185-6. [DOI] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Intracellular calcium and desensitization of acetylcholine receptors. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):447–452. doi: 10.1098/rspb.1980.0106. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transmitter induced calcium entry across the post-synaptic membrane at frog end-plates measured using arsenazo III. J Physiol. 1980 Mar;300:197–212. doi: 10.1113/jphysiol.1980.sp013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]


