Abstract
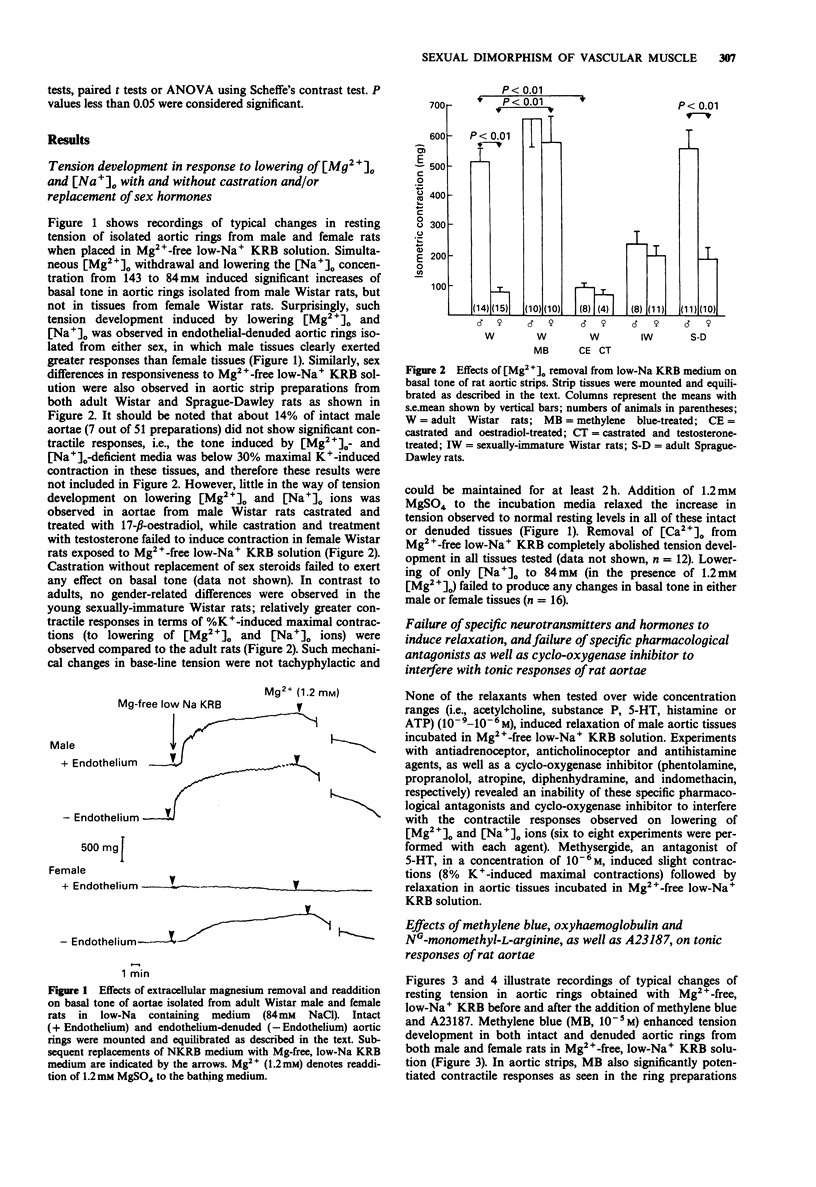
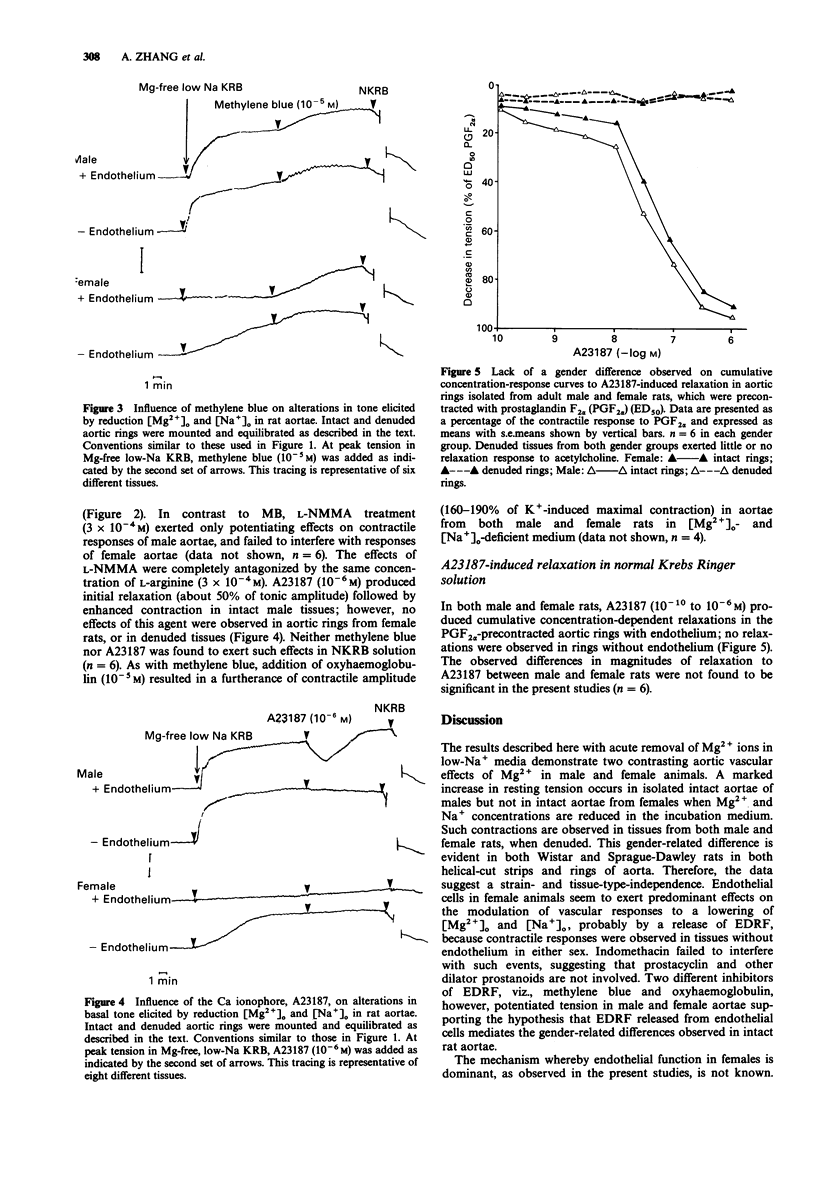
1. In isolated aortae of the male rat [Mg2+]o withdrawal and concomitant reduction in [Na+]o (to 84 mM) induced significant increases of basal tone, but, surprisingly, this did not occur in intact aortae removed from female rats. Such tension development, however, was observed in endothelium-denuded aortic preparations from both sexes. These observed gender-related differences were not dependent on animal strain or types of tissue preparations. 2. No tension development was observed in aortae obtained from castrated males treated with oestradiol. Aortic tissues of sexually-immature male and female rats exhibited marked tension development when exposed to 0 mM [Mg2+]o and low [Na+]o. 3. Tension development in Mg(2+)-free, low-Na+ media was not tachyphylactic and completely dependent on extracellular Ca2+; addition of 1.2 mM Mg2+ to the Mg2+ and Na(+)-deficient incubation media relaxed the increase in tension to a normal basal level. 4. Two known endothelial-derived relaxant factor (EDRF) inhibitors, methylene blue and haemoglobin, induced tension development in female aortae with intact endothelium exposed to Mg(2+)-Na+ deficient media, while use of a specific inhibitor of EDRF-derived nitric oxide, viz., NG-monomethyl-L-arginine (L-NMMA), resulted in potentiation of tension development in male, but not in female, aortae. This effect of L-NMMA was antagonized by L-arginine. 5. The Ca ionophore, A23187, partially relaxed contractile responses in male aortae (with intact endothelium) which were followed by potentiated contractions. Endothelium-dependent vasodilator responses to A23187 (10(-10)-10(-6) M) of aortic rings from male or female rats in normal Krebs-Ringer bicarbonate solution were not different.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Altura B. M., Altura B. T. Differential effects of substrate depletion on drug-induced contractions of rabbit aorta. Am J Physiol. 1970 Dec;219(6):1698–1705. doi: 10.1152/ajplegacy.1970.219.6.1698. [DOI] [PubMed] [Google Scholar]
- Altura B. M., Altura B. T. Magnesium and contraction of arterial smooth muscle. Microvasc Res. 1974 Mar;7(2):145–155. doi: 10.1016/0026-2862(74)90001-6. [DOI] [PubMed] [Google Scholar]
- Altura B. M., Altura B. T. New perspectives on the role of magnesium in the pathophysiology of the cardiovascular system. I. Clinical aspects. Magnesium. 1985;4(5-6):226–244. [PubMed] [Google Scholar]
- Altura B. M., Edgarian H., Altura B. T. Differential effects of ethanol and mannitol on contraction of arterial smooth muscle. J Pharmacol Exp Ther. 1976 May;197(2):352–361. [PubMed] [Google Scholar]
- Altura B. T., Zhang A. M., Altura B. M. Sodium-calcium exchange mechanism in vascular smooth muscle tissue as revealed by manipulating external magnesium. Magnes Trace Elem. 1990;9(3):163–175. [PubMed] [Google Scholar]
- Burnstock G. Mechanisms of interaction of peptide and nonpeptide vascular neurotransmitter systems. J Cardiovasc Pharmacol. 1987;10 (Suppl 12):S74–S81. [PubMed] [Google Scholar]
- Caplan L. R., Gorelick P. B., Hier D. B. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986 Jul-Aug;17(4):648–655. doi: 10.1161/01.str.17.4.648. [DOI] [PubMed] [Google Scholar]
- Colburn P., Buonassisi V. Estrogen-binding sites in endothelial cell cultures. Science. 1978 Sep 1;201(4358):817–819. doi: 10.1126/science.684408. [DOI] [PubMed] [Google Scholar]
- Cox R. H., Fischer G. M. Effects of sex hormones on the passive mechanical properties of rat carotid artery. Blood Vessels. 1978;15(4):266–276. doi: 10.1159/000158172. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982 Oct;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Fischer G. M., Swain M. L. Effect of sex hormones on blood pressure and vascular connective tissue in castrated and noncastrated male rats. Am J Physiol. 1977 Jun;232(6):H617–H621. doi: 10.1152/ajpheart.1977.232.6.H617. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Gisclard V., Miller V. M., Vanhoutte P. M. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988 Jan;244(1):19–22. [PubMed] [Google Scholar]
- Gold M. E., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G., Ignarro L. J. Antagonistic modulatory roles of magnesium and calcium on release of endothelium-derived relaxing factor and smooth muscle tone. Circ Res. 1990 Feb;66(2):355–366. doi: 10.1161/01.res.66.2.355. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Coulson P. B. Estrogen receptors and effects of estrogen on membrane electrical properties of coronary vascular smooth muscle. J Cell Physiol. 1979 Aug;100(2):375–382. doi: 10.1002/jcp.1041000218. [DOI] [PubMed] [Google Scholar]
- Kishi Y., Numano F. A study of the mechanism of estrogen as an antiatherosclerotic: the inhibitory effect of estrogen on A23187-induced contraction of the aortic wall. Mech Ageing Dev. 1982 Feb;18(2):115–123. doi: 10.1016/0047-6374(82)90081-1. [DOI] [PubMed] [Google Scholar]
- Ku D. D., Ann H. S. Magnesium deficiency produces endothelium-dependent vasorelaxation in canine coronary arteries. J Pharmacol Exp Ther. 1987 Jun;241(3):961–966. [PubMed] [Google Scholar]
- Lerner D. J., Kannel W. B. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986 Feb;111(2):383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- Long C. J., Stone T. W. The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels. 1985;22(4):205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Maddox Y. T., Falcon J. G., Ridinger M., Cunard C. M., Ramwell P. W. Endothelium-dependent gender differences in the response of the rat aorta. J Pharmacol Exp Ther. 1987 Feb;240(2):392–395. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl P. K., Cragoe E. J., Jr, Trumble M. J., Kaczorowski G. J. Inhibition of Na+/Ca2+ exchange in membrane vesicle and papillary muscle preparations from guinea pig heart by analogs of amiloride. Proc Natl Acad Sci U S A. 1984 May;81(10):3238–3242. doi: 10.1073/pnas.81.10.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Cragoe E. J., Jr, Smith L. Na+/Ca2+ antiport in cultured arterial smooth muscle cells. Inhibition by magnesium and other divalent cations. J Biol Chem. 1987 Sep 5;262(25):11988–11994. [PubMed] [Google Scholar]
- Smith J. B., Zheng T., Smith L. Relationship between cytosolic free Ca2+ and Na+-Ca2+ exchange in aortic muscle cells. Am J Physiol. 1989 Jan;256(1 Pt 1):C147–C154. doi: 10.1152/ajpcell.1989.256.1.C147. [DOI] [PubMed] [Google Scholar]
- Turlapaty P. D., Altura B. M. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science. 1980 Apr 11;208(4440):198–200. doi: 10.1126/science.7361117. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Goshima K. Kinetic studies on sodium-dependent calcium uptake by myocardial cells and neuroblastoma cells in culture. Biochim Biophys Acta. 1981 Mar 20;642(1):158–172. doi: 10.1016/0005-2736(81)90146-2. [DOI] [PubMed] [Google Scholar]
- Williams J. K., Adams M. R., Klopfenstein H. S. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990 May;81(5):1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Bunting P. B., Baskin E. P., Wallace A. A. Decreased endothelium-dependent relaxation in New Zealand genetic hypertensive rats. J Hypertens. 1984 Oct;2(5):541–545. doi: 10.1097/00004872-198410000-00015. [DOI] [PubMed] [Google Scholar]


