Abstract
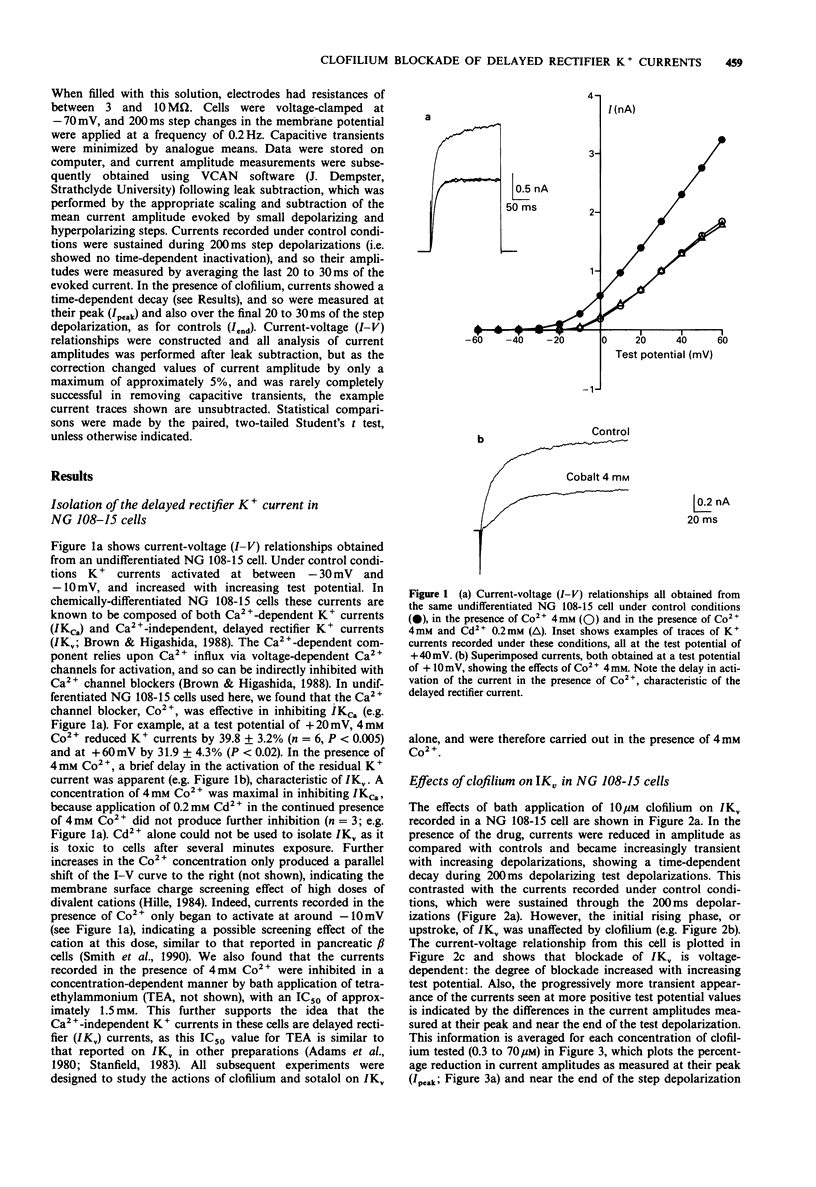
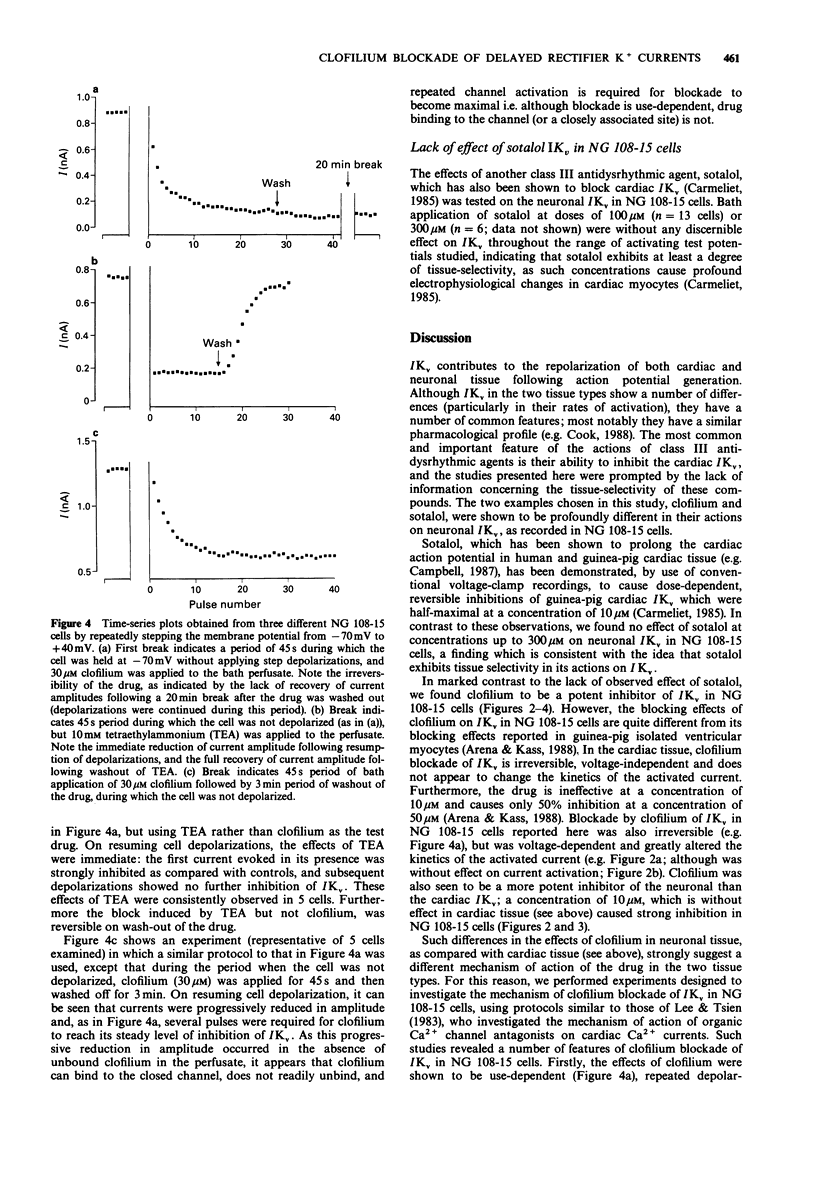
1. The whole-cell patch-clamp technique was used to examine the effects of the class III antidysrhythmic agent, clofilium, on voltage-activated delayed rectifier K+ currents (IKv) in undifferentiated mouse neuroblastoma x rat glioma hybrid (NG 108-15) cells. Ca(2+)-activated K+ currents also seen in these cells were abolished by bath application of 4 mM Co2+. 2. Bath application of clofilium (0.3 to 70 microM) caused dose-dependent, irreversible inhibition of IKv in these cells. Under control conditions, activated currents were sustained during 200 ms depolarizing steps, but in the presence of clofilium, or after its wash-out, currents were reduced in amplitude and showed a time-dependent decay. 3. Clofilium blockade of IKv was voltage-dependent; the degree of current inhibition increased with increasing depolarizations. The transient nature of IKv seen in the presence of clofilium was also more apparent at higher test potentials. 4. The effects of clofilium were use-dependent: when cells were left unstimulated during drug application, and then depolarizations were resumed, several pulses were required for clofilium blockade to reach a steady level. Similar results were obtained post-clofilium, when cells were unstimulated during application and then removal of clofilium, suggesting that although the blocking action of the drug was use-dependent, it bound to the closed, delayed rectifier K+ channel. 5. High concentrations (100 or 300 microM) of sotalol, another class III antidysrhythmic agent, were without discernible effects on IKv in NG 108-15 cells. 6. The effects of clofilium on a neuronal IKv described here, and its possible mechanism of action, are compared with previously reported effects of clofilium on the cardiac IKv.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Arena J. P., Kass R. S. Block of heart potassium channels by clofilium and its tertiary analogs: relationship between drug structure and type of channel blocked. Mol Pharmacol. 1988 Jul;34(1):60–66. [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Voltage- and calcium-activated potassium currents in mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:149–165. doi: 10.1113/jphysiol.1988.sp016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T. J. Cellular electrophysiological effects of D- and DL-sotalol in guinea-pig sinoatrial node, atrium and ventricle and human atrium: differential tissue sensitivity. Br J Pharmacol. 1987 Mar;90(3):593–599. doi: 10.1111/j.1476-5381.1987.tb11210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Electrophysiologic and voltage clamp analysis of the effects of sotalol on isolated cardiac muscle and Purkinje fibers. J Pharmacol Exp Ther. 1985 Mar;232(3):817–825. [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Nettleton J., Castle N. A., Wang G. K. Block of single batrachotoxin-activated Na+ channels by clofilium. Mol Pharmacol. 1991 Mar;39(3):352–358. [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Bokvist K., Arkhammar P., Berggren P. O., Rorsman P. Delayed rectifying and calcium-activated K+ channels and their significance for action potential repolarization in mouse pancreatic beta-cells. J Gen Physiol. 1990 Jun;95(6):1041–1059. doi: 10.1085/jgp.95.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]



