Abstract
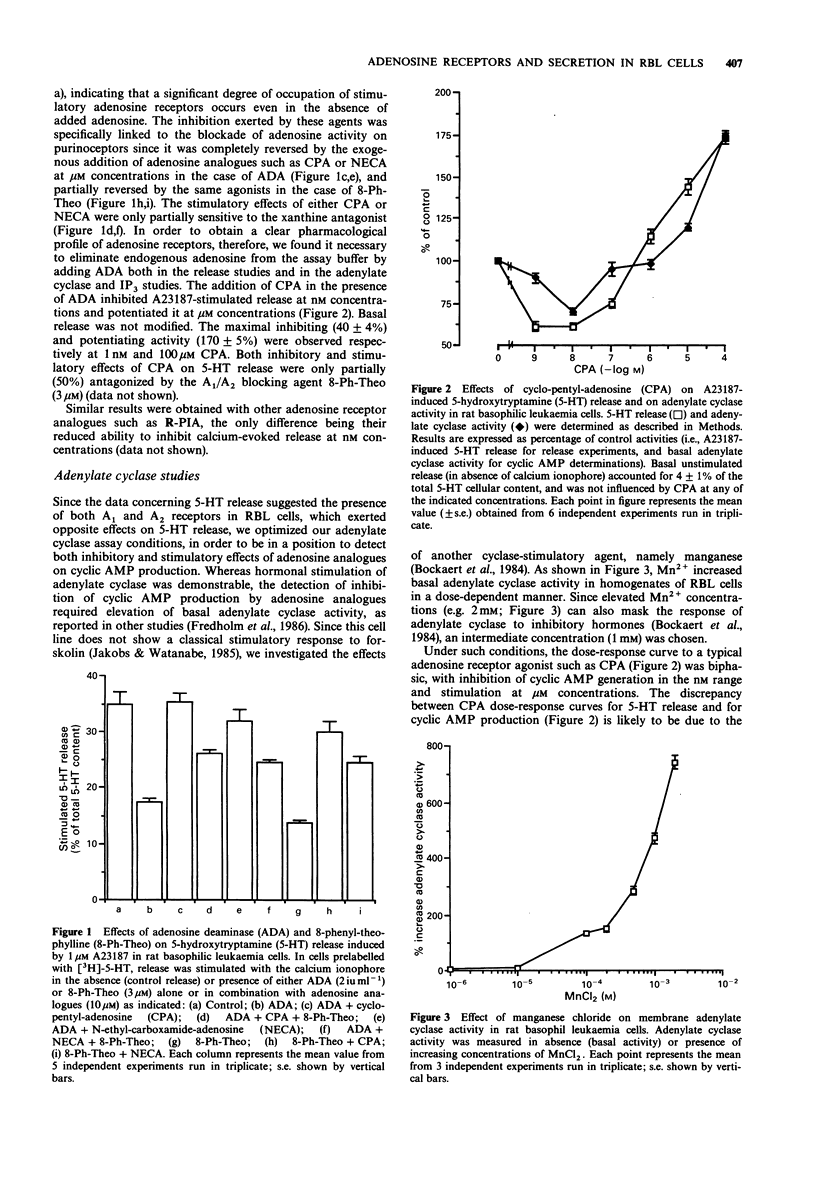
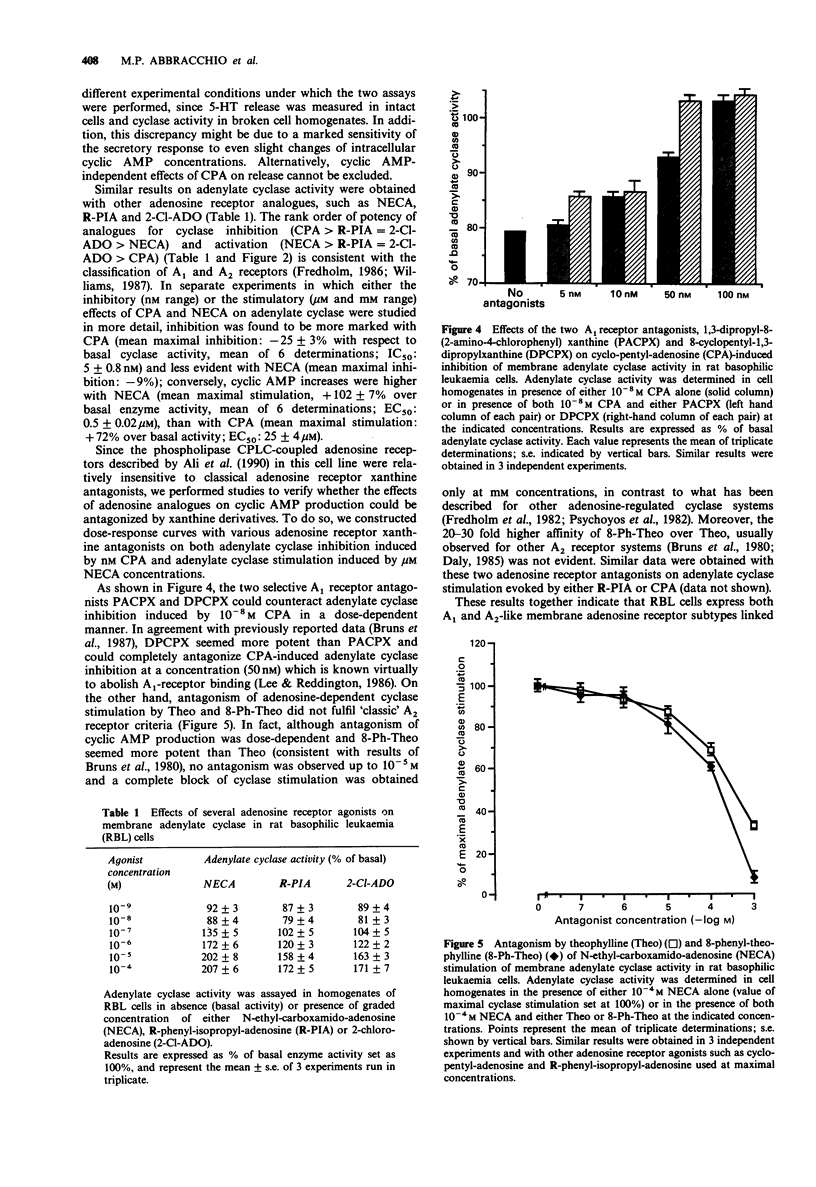
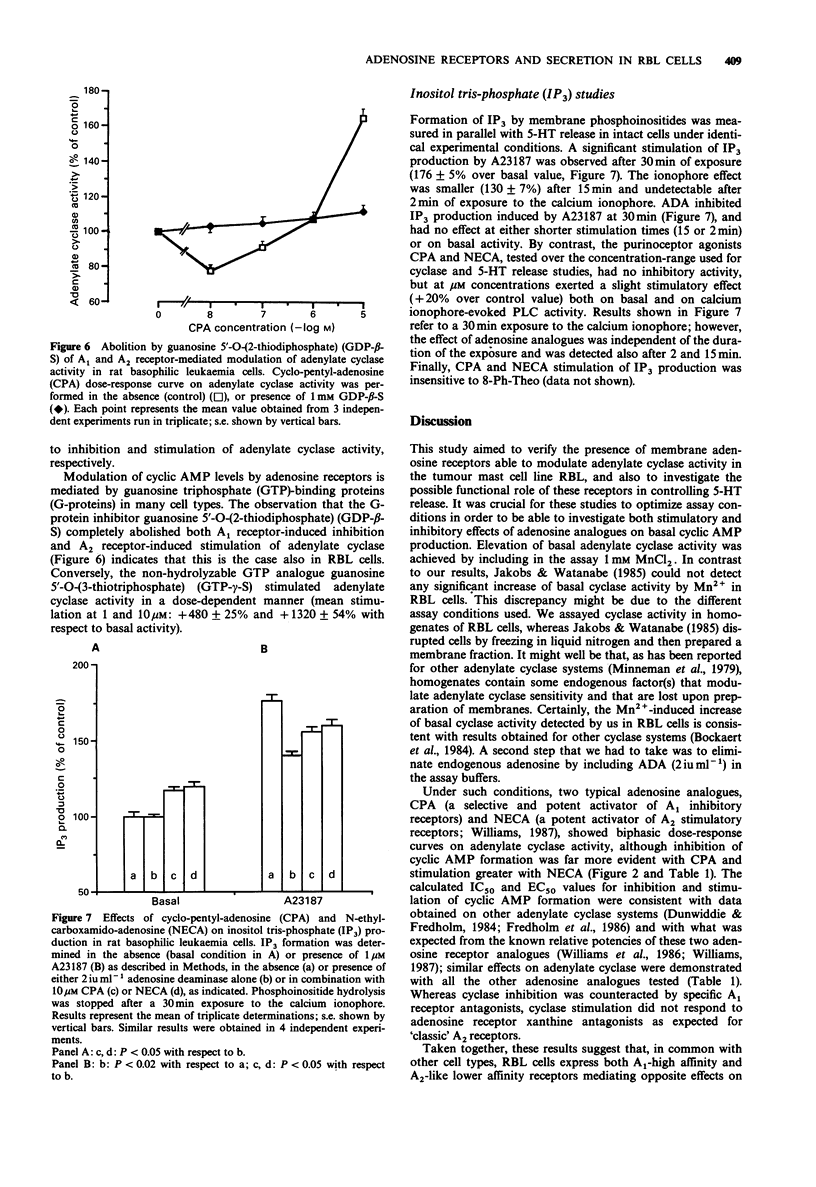
1. The presence of adenosine receptors linked to adenylate cyclase activity and their functional role in calcium-evoked 5-hydroxytryptamine (5-HT) release was investigated in rat basophilic leukaemia (RBL) cells, a widely used model for studying the molecular mechanisms responsible for stimulus-secretion coupling. 2. In [3H]-5-HT-loaded cells triggered to release by the calcium ionophore A23187, a biphasic modulation of 5-HT secretion was induced by adenosine analogues, with inhibition of stimulated release at nM and potentiation at microM concentrations, suggesting the presence of adenosine receptor subtypes mediating opposite effects on calcium-dependent release. This was also confirmed by results obtained with other agents interfering with adenosine pharmacology, such as adenosine deaminase and the non-selective A1/A2 antagonist 8-phenyl-theophylline. 3. Similar biphasic dose-response curves were obtained with a variety of adenosine analogues on basal adenylate cyclase activity in RBL cells, with inhibition and stimulation of adenosine 3':5'-cyclic monophosphate (cyclic AMP) production at nM and microM concentrations, respectively. The rank order of potency of adenosine analogues for inhibition and stimulation of adenylate cyclase activity and the involvement of G-proteins in modulation of cyclic AMP levels suggested the presence of cyclase-linked A1 high-affinity and A2-like low-affinity adenosine receptor subtypes. However, the atypical antagonism profile displayed by adenosine receptor xanthine antagonists on cyclase stimulation suggested that the A2-like receptor expressed by RBL cells might represent a novel cyclase-coupled A2 receptor subtype.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbracchio M. P., Cattabeni F., Clementi F., Sher E. Adenosine receptors linked to adenylate cyclase activity in human neuroblastoma cells: modulation during cell differentiation. Neuroscience. 1989;30(3):819–825. doi: 10.1016/0306-4522(89)90173-5. [DOI] [PubMed] [Google Scholar]
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J., Cantau B., Sebben-Perez M. Hormonal inhibition of adenylate cyclase. A crucial role for Mg2+. Mol Pharmacol. 1984 Sep;26(2):180–186. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hartman J. D., Hays S. J., Huang C. C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Church M. K., Holgate S. T., Hughes P. J. Adenosine inhibits and potentiates IgE-dependent histamine release from human basophils by an A2-receptor mediated mechanism. Br J Pharmacol. 1983 Dec;80(4):719–726. doi: 10.1111/j.1476-5381.1983.tb10063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M., Caldwell K. K., Boyajian C. L., Petcoff D. W., Schlegel W. Adenosine A1 receptors inhibit both adenylate cyclase activity and TRH-activated Ca2+ channels by a pertussis toxin-sensitive mechanism in GH3 cells. Cell Signal. 1989;1(1):85–97. doi: 10.1016/0898-6568(89)90023-5. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:29–46. [PubMed] [Google Scholar]
- De Matteis M. A., Di Tullio G., Buccione R., Luini A. Characterization of calcium-triggered secretion in permeabilized rat basophilic leukemia cells. Possible role of vectorially acting G proteins. J Biol Chem. 1991 Jun 5;266(16):10452–10460. [PubMed] [Google Scholar]
- Delahunty T. M., Cronin M. J., Linden J. Regulation of GH3-cell function via adenosine A1 receptors. Inhibition of prolactin release, cyclic AMP production and inositol phosphate generation. Biochem J. 1988 Oct 1;255(1):69–77. doi: 10.1042/bj2550069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Fredholm B. B. Adenosine receptors mediating inhibitory electrophysiological responses in rat hippocampus are different from receptors mediating cyclic AMP accumulation. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jul;326(4):294–301. doi: 10.1007/BF00501433. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988 Apr;9(4):130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Jonzon B., Lindgren E., Lindström K. Adenosine receptors mediating cyclic AMP production in the rat hippocampus. J Neurochem. 1982 Jul;39(1):165–175. doi: 10.1111/j.1471-4159.1982.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Jonzon B., Lindström K. Effect of adenosine receptor agonists and other compounds on cyclic AMP accumulation in forskolin-treated hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol. 1986 Feb;332(2):173–178. doi: 10.1007/BF00511409. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Guild S., Frey E. A., Pocotte S. L., Kebabian J. W. Adenosine 3',5'-cyclic monophosphate-mediated enhancement of calcium-evoked prolactin release from electrically permeabilised 7315c tumour cells. Br J Pharmacol. 1988 Jul;94(3):737–744. doi: 10.1111/j.1476-5381.1988.tb11583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs K. H., Watanabe Y. Stimulation and inhibition of rat basophilic leukemia cell adenylate cyclase by forskolin. Biochim Biophys Acta. 1985 Sep 30;846(3):356–363. doi: 10.1016/0167-4889(85)90006-0. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Fyles J. M., Howell S. L. Regulation of insulin secretion by cAMP in rat islets of Langerhans permeabilised by high-voltage discharge. FEBS Lett. 1986 Sep 15;205(2):205–209. doi: 10.1016/0014-5793(86)80898-5. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Reddington M. 1,3-Dipropyl-8-cyclopentylxanthine (DPCPX) inhibition of [3H]N-ethylcarboxamidoadenosine (NECA) binding allows the visualization of putative non-A1 adenosine receptors. Brain Res. 1986 Mar 19;368(2):394–398. doi: 10.1016/0006-8993(86)90589-5. [DOI] [PubMed] [Google Scholar]
- Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone G., Cirillo R., Genovese A., Marino O., Quattrin S. Human basophil/mast cell releasability. VII. Heterogeneity of the effect of adenosine on mediator secretion. Life Sci. 1989;45(19):1745–1754. doi: 10.1016/0024-3205(89)90513-4. [DOI] [PubMed] [Google Scholar]
- Marquardt D. L., Gruber H. E., Wasserman S. I. Adenosine release from stimulated mast cells. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6192–6196. doi: 10.1073/pnas.81.19.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt D. L., Walker L. L. Modulation of mast cell responses to adenosine by agents that alter protein kinase C activity. Biochem Pharmacol. 1990 Jun 15;39(12):1929–1934. doi: 10.1016/0006-2952(90)90611-n. [DOI] [PubMed] [Google Scholar]
- McCloskey M. A. Cholera toxin potentiates IgE-coupled inositol phospholipid hydrolysis and mediator secretion by RBL-2H3 cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7260–7264. doi: 10.1073/pnas.85.19.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneman K. P., Hegstrand L. R., Molinoff P. B. The pharmacological specificity of beta-1 and beta-2 adrenergic receptors in rat heart and lung in vitro. Mol Pharmacol. 1979 Jul;16(1):21–33. [PubMed] [Google Scholar]
- Narasimhan V., Holowka D., Fewtrell C., Baird B. Cholera toxin increases the rate of antigen-stimulated calcium influx in rat basophilic leukemia cells. J Biol Chem. 1988 Dec 25;263(36):19626–19632. [PubMed] [Google Scholar]
- Psychoyos S., Dove J., Strowbridge B., Nusynowitz I. Highly activatable adenylate cyclase in [2-3H]adenine-prelabeled vesicles prepared from guinea pig cerebral cortex by a simplified procedure. J Neurochem. 1982 May;38(5):1437–1445. doi: 10.1111/j.1471-4159.1982.tb07923.x. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Spignoli G., Pedata F., Pepeu G. A1 and A2 adenosine receptors modulate acetylcholine release from brain slices. Eur J Pharmacol. 1984 Jan 27;97(3-4):341–342. doi: 10.1016/0014-2999(84)90475-8. [DOI] [PubMed] [Google Scholar]
- Williams M., Braunwalder A., Erickson T. J. Evaluation of the binding of the A-1 selective adenosine radioligand, cyclopentyladenosine (CPA), to rat brain tissue. Naunyn Schmiedebergs Arch Pharmacol. 1986 Feb;332(2):179–183. doi: 10.1007/BF00511410. [DOI] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]



