Abstract
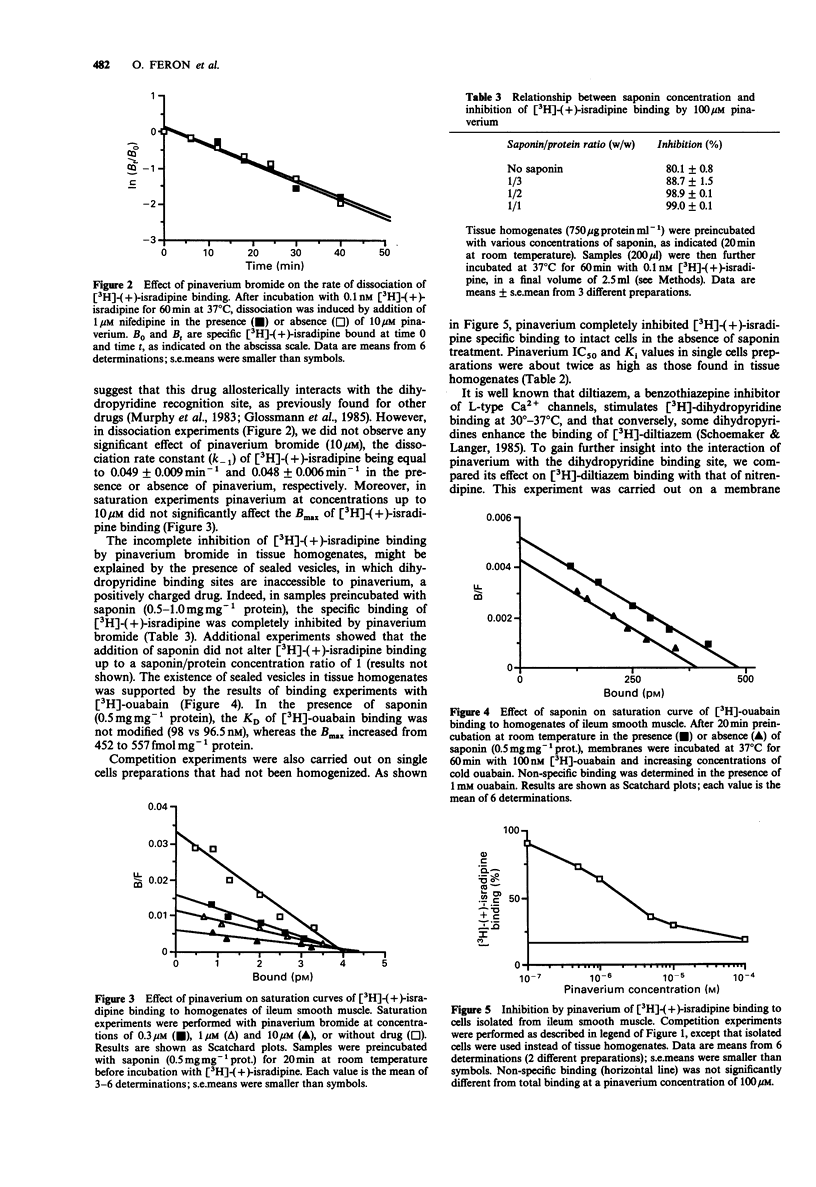
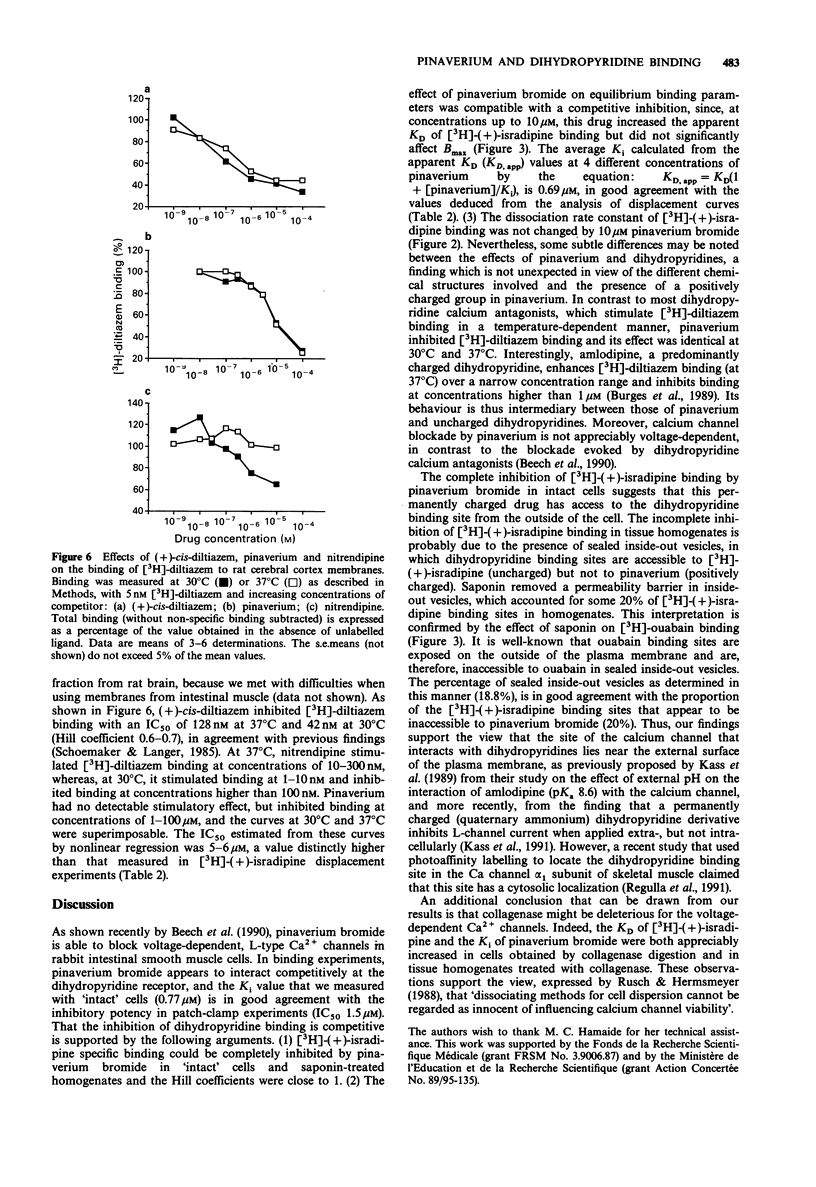
1. The interaction of pinaverium bromide, a quaternary ammonium compound, with binding sites for (L-type) calcium channel blockers was investigated in rat ileum smooth muscle. 2. Pinaverium inhibited [3H]-(+)-PN200-110 ([3H]-(+)-isradipine) specific binding to tissue homogenates incompletely (Ki 0.38 microM; maximal inhibition 80%). In contrast, binding to single cell preparations (obtained by collagenase treatment) and to saponin-treated homogenates was completely inhibited. These data are compatible with the view that, in untreated homogenates, 20% of [3H]-(+)-isradipine binding sites are not accessible to pinaverium because it is associated with sealed inside-out vesicles. 3. Pinaverium bromide increased the apparent KD of [3H]-(+)-isradipine binding to saponin-treated homogenates but did not significantly affect the Bmax value. Moreover, the dissociation rate constant of [3H]-(+)-isradipine binding was not changed by pinaverium. These data suggest that pinaverium interacts with the dihydropyridine binding site in a competitive manner. However, in contrast to uncharged dihydropyridine calcium antagonists, pinaverium inhibited, rather than stimulated, [3H]-diltiazem binding to rat brain membranes (at 30-37 degrees C). 4. Although Bmax values of [3H]-(+)-isradipine were similar in homogenates prepared from tissue and cells (collagenase-treated), the KD value was significantly higher in cell homogenates (166 vs 95 pM). Similarly, the Ki value of pinaverium was higher in cell preparations than in tissue homogenates (0.77 vs 0.38 microM). Thus, collagenase can significantly modify the dihydropyridine recognition site.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner A., Drack E., Halter F., Scheurer U. Effects of pinaverium bromide and verapamil on the motility of the rat isolated colon. Br J Pharmacol. 1985 Sep;86(1):89–94. doi: 10.1111/j.1476-5381.1985.tb09438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., MacKenzie I., Bolton T. B., Christen M. O. Effects of pinaverium on voltage-activated calcium channel currents of single smooth muscle cells isolated from the longitudinal muscle of the rabbit jejunum. Br J Pharmacol. 1990 Feb;99(2):374–378. doi: 10.1111/j.1476-5381.1990.tb14711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen M. O. Action of pinaverium bromide, a calcium-antagonist, on gastrointestinal motility disorders. Gen Pharmacol. 1990;21(6):821–825. doi: 10.1016/0306-3623(90)90439-s. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Himpens B., Casteels R. Effect of pinaverium bromide on electrical and mechanical activity of smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(1):72–77. doi: 10.1007/BF00498831. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Kass R. S., Arena J. P., Chin S. Block of L-type calcium channels by charged dihydropyridines. Sensitivity to side of application and calcium. J Gen Physiol. 1991 Jul;98(1):63–75. doi: 10.1085/jgp.98.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Gould R. J., Largent B. L., Snyder S. H. A unitary mechanism of calcium antagonist drug action. Proc Natl Acad Sci U S A. 1983 Feb;80(3):860–864. doi: 10.1073/pnas.80.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel F., Godfraind T. Heterogeneity of ouabain specific binding sites and (Na+ + K+)-ATPase inhibition in microsomes from rat heart. Biochem Pharmacol. 1984 Jan 1;33(1):47–53. doi: 10.1016/0006-2952(84)90369-1. [DOI] [PubMed] [Google Scholar]
- Regulla S., Schneider T., Nastainczyk W., Meyer H. E., Hofmann F. Identification of the site of interaction of the dihydropyridine channel blockers nitrendipine and azidopine with the calcium-channel alpha 1 subunit. EMBO J. 1991 Jan;10(1):45–49. doi: 10.1002/j.1460-2075.1991.tb07919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch N. J., Hermsmeyer K. Measurement of whole-cell calcium current in voltage-clamped vascular muscle cells. Mol Cell Biochem. 1988 Mar-Apr;80(1-2):87–93. doi: 10.1007/BF00231007. [DOI] [PubMed] [Google Scholar]
- Schoemaker H., Langer S. Z. [3H]diltiazem binding to calcium channel antagonists recognition sites in rat cerebral cortex. Eur J Pharmacol. 1985 May 8;111(2):273–277. doi: 10.1016/0014-2999(85)90768-x. [DOI] [PubMed] [Google Scholar]
- Takayanagi I., Koike K., Hiruta T. Interactions of muscarinic drugs with their receptors in single cells of guinea-pig taenia caecum. J Pharm Pharmacol. 1986 Jun;38(6):476–478. doi: 10.1111/j.2042-7158.1986.tb04615.x. [DOI] [PubMed] [Google Scholar]
- Wibo M., DeRoth L., Godfraind T. Pharmacologic relevance of dihydropyridine binding sites in membranes from rat aorta: kinetic and equilibrium studies. Circ Res. 1988 Jan;62(1):91–96. doi: 10.1161/01.res.62.1.91. [DOI] [PubMed] [Google Scholar]



