Abstract
1. Hippocampal slices have been used to assess the sensitivity of the CNS to adenosine and gamma-aminobutyric acid (GABA) in diabetes. The effects of adenosine, 2-chloroadenosine, GABA, muscimol and baclofen were studied on orthodromic synaptic potentials recorded in the CA1 region of slices taken from normal rats or animals made diabetic by the injection of streptozotocin. 2. In diabetic animals the sensitivity to adenosine was increased 4 fold compared with normal rats. The potency of 2-chloroadenosine was unchanged. 3. The nucleoside transport inhibitor, hydroxynitrobenzylthioinosine (HNBTI), increased the potency of adenosine in slices from normal rats but not in slices from diabetic rats. 4. No change was observed in the potency of GABA or muscimol, although a small but significant decrease was detected in the EC50 value for baclofen. 5. Treatment of diabetic animals with insulin restored the potency of adenosine to control levels. 6. It is concluded that the diabetic state is accompanied by substantial changes of adenosine sensitivity due to the loss of nucleoside uptake processes. Secondary neurochemical changes following from this in human diabetic patients may contribute to the reported behavioural changes.
Full text
PDF
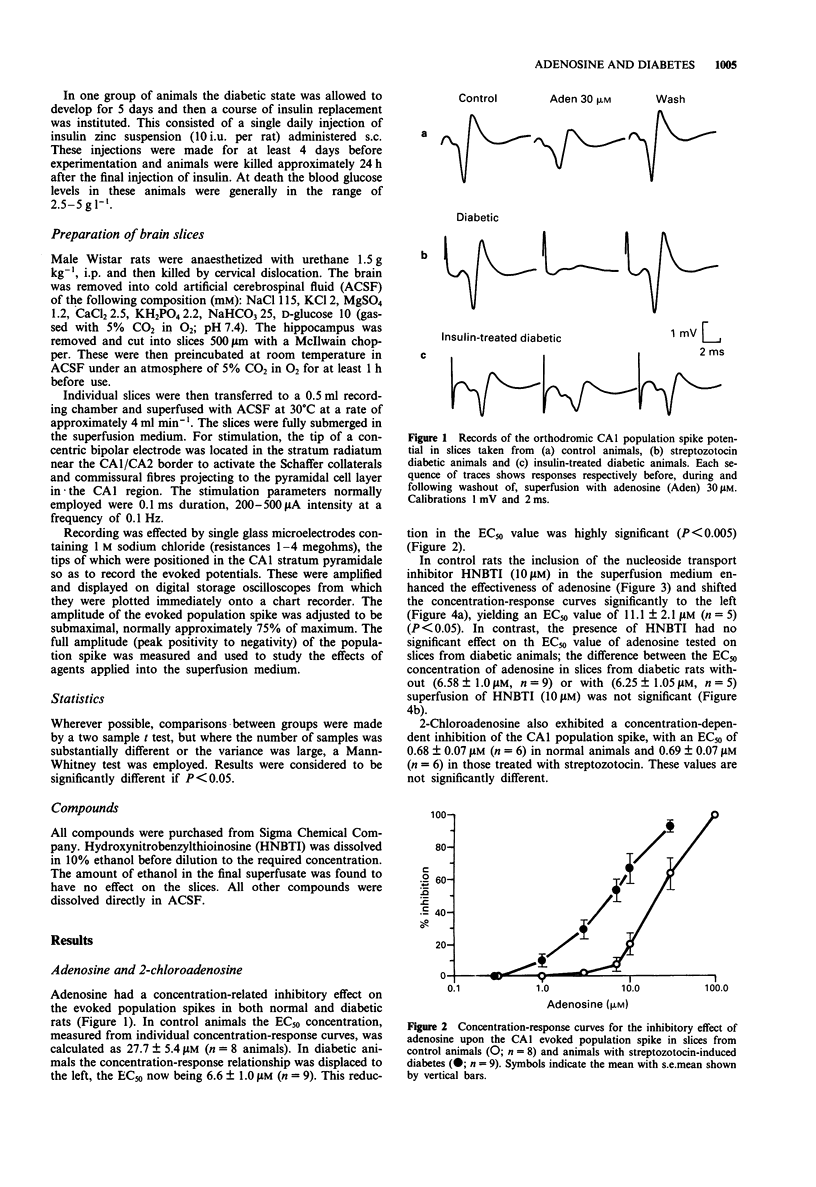
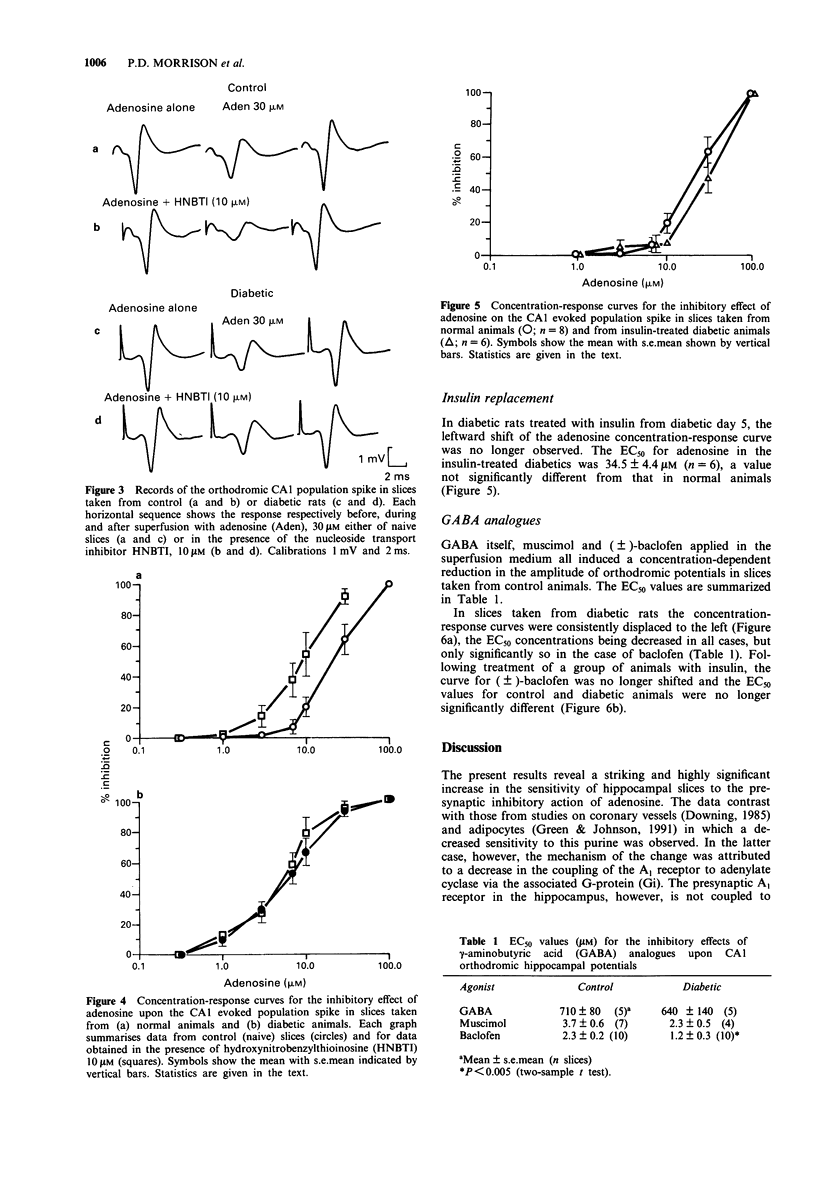
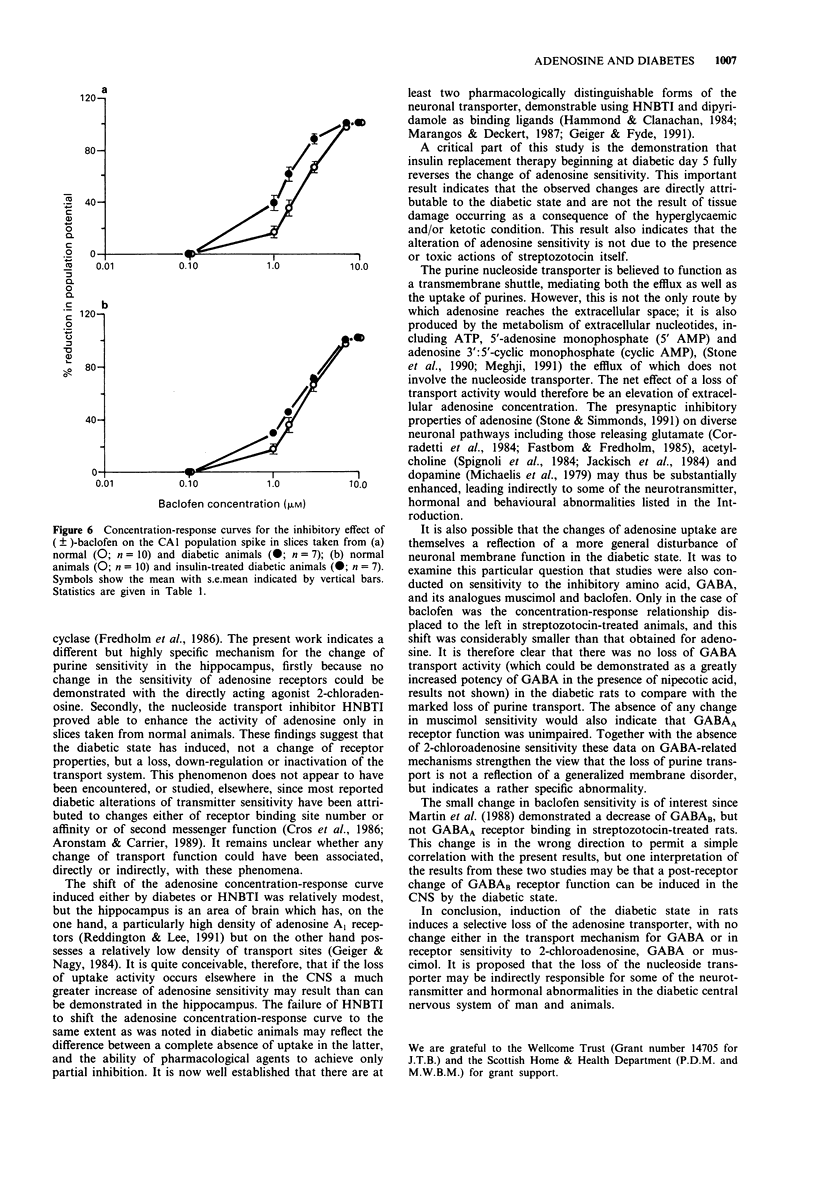

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends J., Wagner M. L., Willms B. L. Cholinergic muscarinic receptor blockade suppresses arginine- and exercise-induced growth hormone secretion in type I diabetic subjects. J Clin Endocrinol Metab. 1988 Feb;66(2):389–394. doi: 10.1210/jcem-66-2-389. [DOI] [PubMed] [Google Scholar]
- Aronstam R. S., Carrier G. O. Insulin prevention of altered muscarinic receptor-G protein coupling in diabetic rat atria. Diabetes. 1989 Dec;38(12):1611–1616. doi: 10.2337/diab.38.12.1611. [DOI] [PubMed] [Google Scholar]
- BRUCH H. Physiologic and psychologic interrelationships in diabetes in children. Psychosom Med. 1949 Jul-Aug;11(4):200–210. doi: 10.1097/00006842-194907000-00002. [DOI] [PubMed] [Google Scholar]
- Berlin I., Grimaldi A., Bosquet F., Puech A. J. Decreased beta-adrenergic sensitivity in insulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1986 Jul;63(1):262–265. doi: 10.1210/jcem-63-1-262. [DOI] [PubMed] [Google Scholar]
- Bitar M., Koulu M., Rapoport S. I., Linnoila M. Diabetes-induced alteration in brain monoamine metabolism in rats. J Pharmacol Exp Ther. 1986 Feb;236(2):432–437. [PubMed] [Google Scholar]
- Carrier G. O., Aronstam R. S. Altered muscarinic receptor properties and function in the heart in diabetes. J Pharmacol Exp Ther. 1987 Aug;242(2):531–535. [PubMed] [Google Scholar]
- Chu P. C., Lin M. T., Shian L. R., Leu S. Y. Alterations in physiologic functions and in brain monoamine content in streptozocin-diabetic rats. Diabetes. 1986 Apr;35(4):481–485. doi: 10.2337/diab.35.4.481. [DOI] [PubMed] [Google Scholar]
- Coiro V., Volpi R., Capretti L., Speroni G., Castelli A., Mosti A., Marchesi C., Gardini E., Rossi G., Chiodera P. Simultaneous inhibition by pirenzepine of the GH responses to GnRH and TRH in insulin-dependent diabetics and in patients with major depression. Acta Endocrinol (Copenh) 1989 Feb;120(2):143–148. doi: 10.1530/acta.0.1200143. [DOI] [PubMed] [Google Scholar]
- Corradetti R., Lo Conte G., Moroni F., Passani M. B., Pepeu G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur J Pharmacol. 1984 Sep 3;104(1-2):19–26. doi: 10.1016/0014-2999(84)90364-9. [DOI] [PubMed] [Google Scholar]
- Cros G. H., Chanez P. O., Michel A., McNeill J. H., Serrano J. J. Cardiac beta-adrenergic receptors in diabetic rats: alteration of guanyl nucleotide regulation. J Pharmacol. 1986 Oct-Dec;17(4):595–600. [PubMed] [Google Scholar]
- Downing S. E. Restoration of coronary dilator action of adenosine in experimental diabetes. Am J Physiol. 1985 Jul;249(1 Pt 2):H102–H107. doi: 10.1152/ajpheart.1985.249.1.H102. [DOI] [PubMed] [Google Scholar]
- Drukarch B., Schepens E., Heimans J. J., Stoof J. C. Alpha 2-adrenoceptor-mediated modulation of noradrenaline release is decreased in vas deferens but not in cerebral cortex of diabetic rats. J Neurol Sci. 1989 Jul;91(3):293–299. doi: 10.1016/0022-510x(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Fastbom J., Fredholm B. B. Inhibition of [3H] glutamate release from rat hippocampal slices by L-phenylisopropyladenosine. Acta Physiol Scand. 1985 Sep;125(1):121–123. doi: 10.1111/j.1748-1716.1985.tb07698.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Fastbom J., Lindgren E. Effects of N-ethylmaleimide and forskolin on glutamate release from rat hippocampal slices. Evidence that prejunctional adenosine receptors are linked to N-proteins, but not to adenylate cyclase. Acta Physiol Scand. 1986 Jul;127(3):381–386. doi: 10.1111/j.1748-1716.1986.tb07918.x. [DOI] [PubMed] [Google Scholar]
- Geiger J. D., Nagy J. I. Heterogeneous distribution of adenosine transport sites labelled by [3H]nitrobenzylthioinosine in rat brain: an autoradiographic and membrane binding study. Brain Res Bull. 1984 Nov;13(5):657–666. doi: 10.1016/0361-9230(84)90198-9. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Synder S. H. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982 Sep;2(9):1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A., Johnson J. L. Evidence for impaired coupling of receptors to Gi protein in adipocytes from streptozocin-induced diabetic rats. Diabetes. 1991 Jan;40(1):88–94. doi: 10.2337/diab.40.1.88. [DOI] [PubMed] [Google Scholar]
- Hammond J. R., Clanachan A. S. Heterogeneity of high affinity nitrobenzylthioinosine binding sites in mammalian cortical membranes: multiple forms of central nervous system nucleoside transporters? Can J Physiol Pharmacol. 1984 Aug;62(8):961–963. doi: 10.1139/y84-161. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L. A., Wozniak K. M., Durcan M. J., Linnoila M. Behavior of streptozotocin-diabetic mice in tests of exploration, locomotion, anxiety, depression and aggression. Physiol Behav. 1990 Sep;48(3):429–433. doi: 10.1016/0031-9384(90)90339-6. [DOI] [PubMed] [Google Scholar]
- Ingebretsen C. G., Hawelu-Johnson C., Ingebretsen W. R., Jr Alloxan-induced diabetes reduces beta-adrenergic receptor number without affecting adenylate cyclase in rat ventricular membranes. J Cardiovasc Pharmacol. 1983 May-Jun;5(3):454–461. doi: 10.1097/00005344-198305000-00018. [DOI] [PubMed] [Google Scholar]
- Jackisch R., Strittmatter H., Kasakov L., Hertting G. Endogenous adenosine as a modulator of hippocampal acetylcholine release. Naunyn Schmiedebergs Arch Pharmacol. 1984 Oct;327(4):319–325. doi: 10.1007/BF00506243. [DOI] [PubMed] [Google Scholar]
- Kamei J., Ogawa M., Kasuya Y. Development of supersensitivity to substance P in the spinal cord of the streptozotocin-induced diabetic rats. Pharmacol Biochem Behav. 1990 Feb;35(2):473–475. doi: 10.1016/0091-3057(90)90188-n. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A., Nishio Y., Saeki Y., Kida Y., Kodama M., Shigeta Y. Plasma membrane-specific deficiency in cardiac beta-adrenergic receptor in streptozocin-diabetic rats. Am J Physiol. 1989 Aug;257(2 Pt 1):E127–E132. doi: 10.1152/ajpendo.1989.257.2.E127. [DOI] [PubMed] [Google Scholar]
- Latifpour J., Gousse A., Kondo S., Morita T., Weiss R. M. Effects of experimental diabetes on biochemical and functional characteristics of bladder muscarinic receptors. J Pharmacol Exp Ther. 1989 Jan;248(1):81–88. [PubMed] [Google Scholar]
- Lozovsky D., Saller C. F., Kopin I. J. Dopamine receptor binding is increased in diabetic rats. Science. 1981 Nov 27;214(4524):1031–1033. doi: 10.1126/science.6458088. [DOI] [PubMed] [Google Scholar]
- Marangos P. J., Deckert J. [3H]dipyridamole binding to guinea pig brain membranes: possible heterogeneity of central adenosine uptake sites. J Neurochem. 1987 Apr;48(4):1231–1236. doi: 10.1111/j.1471-4159.1987.tb05651.x. [DOI] [PubMed] [Google Scholar]
- Martin P., Massol J., Pichat P., Puech A. J. Decreased central GABA B receptor binding sites in diabetic rats. Neuropsychobiology. 1988;19(3):146–148. doi: 10.1159/000118451. [DOI] [PubMed] [Google Scholar]
- Michaelis M. L., Michaelis E. K., Myers S. L. Adenosine modulation of synaptosomal dopamine release. Life Sci. 1979 May 28;24(22):2083–2092. doi: 10.1016/0024-3205(79)90082-1. [DOI] [PubMed] [Google Scholar]
- Page M. D., Koppeschaar H. P., Dieguez C., Gibbs J. T., Hall R., Peters J. R., Scanlon M. F. Cholinergic muscarinic receptor blockade with pirenzepine abolishes slow wave sleep-related growth hormone release in young patients with insulin-dependent diabetes mellitus. Clin Endocrinol (Oxf) 1987 Mar;26(3):355–359. doi: 10.1111/j.1365-2265.1987.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Ramanadham S., Tenner T. E., Jr Alterations in the myocardial beta-adrenoceptor system of streptozotocin-diabetic rats. Eur J Pharmacol. 1987 Apr 29;136(3):377–389. doi: 10.1016/0014-2999(87)90311-6. [DOI] [PubMed] [Google Scholar]
- Rowland N., Joyce J. N., Bellush L. L. Stereotyped behavior and diabetes mellitus in rats: reduced behavioral effects of amphetamine and apomorphine and reduced in vivo brain binding of [3H]spiroperidol. Behav Neurosci. 1985 Oct;99(5):831–841. doi: 10.1037//0735-7044.99.5.831. [DOI] [PubMed] [Google Scholar]
- Ryan C., Vega A., Longstreet C., Drash A. Neuropsychological changes in adolescents with insulin-dependent diabetes. J Consult Clin Psychol. 1984 Jun;52(3):335–342. doi: 10.1037//0022-006x.52.3.335. [DOI] [PubMed] [Google Scholar]
- Solomon S. S., Schwartz Y., Rawlinson T. Lipolysis in diabetic adipocytes: differences in response to growth hormone and adenosine. Endocrinology. 1987 Sep;121(3):1056–1060. doi: 10.1210/endo-121-3-1056. [DOI] [PubMed] [Google Scholar]
- Spignoli G., Pedata F., Pepeu G. A1 and A2 adenosine receptors modulate acetylcholine release from brain slices. Eur J Pharmacol. 1984 Jan 27;97(3-4):341–342. doi: 10.1016/0014-2999(84)90475-8. [DOI] [PubMed] [Google Scholar]
- Tang F. Changes in met-enkephalin and beta-endorphin contents in the hypothalamus and the pituitary in diabetic rats: effects of insulin therapy. Clin Exp Pharmacol Physiol. 1989 Feb;16(2):65–75. doi: 10.1111/j.1440-1681.1989.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Himmel C. D. Decreased brain dopamine synthesis rate and increased [3H]spiroperidol binding in streptozotocin-diabetic rats. J Neurochem. 1983 May;40(5):1456–1459. doi: 10.1111/j.1471-4159.1983.tb13590.x. [DOI] [PubMed] [Google Scholar]
- Wald M., Borda E. S., Sterin-Borda L. Alpha-adrenergic supersensitivity and decreased number of alpha-adrenoceptors in heart from acute diabetic rats. Can J Physiol Pharmacol. 1988 Sep;66(9):1154–1160. doi: 10.1139/y88-190. [DOI] [PubMed] [Google Scholar]


