Abstract
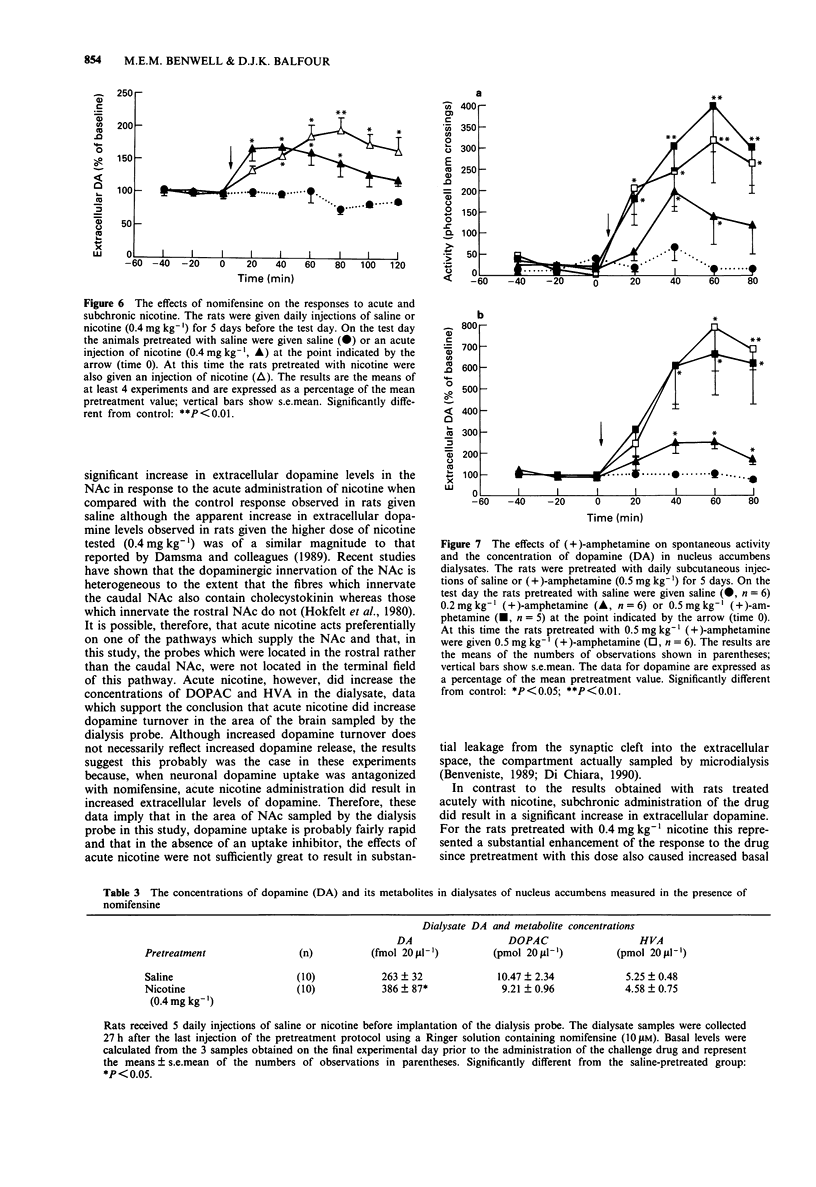
1. The effects of acute and subchronic nicotine and (+)-amphetamine on the extracellular levels of dopamine and its metabolites, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in nucleus accumbens (NAc) have been studied in conscious, freely-moving rats by use of in vivo microdialysis. 2. In rats which had been habituated to the test apparatus for approximately 80 min, the acute subcutaneous (s.c.) administration of nicotine (0.1 or 0.4 mg kg-1) caused a dose-dependent increase (P less than 0.01) in spontaneous activity and evoked significant increases (P less than 0.05) in the extracellular levels of DOPAC and HVA. 3. Measurements made 24 h after the last injection of nicotine showed that pretreatment with the higher doses tested (0.4 mg kg-1) resulted in increased basal levels of dopamine (P less than 0.01) and decreased basal levels of DOPAC (P less than 0.05) in the NAc dialysates. 4. Pretreatment with nicotine (0.1 or 0.4 mg kg-1 daily for 5 days) enhanced the effects of the drug on spontaneous locomotor activity and enhanced the effects of the drug on extracellular levels of dopamine to the extent that the response became significant (P less than 0.05). 5. If a dopamine uptake inhibitor, nomifensine, was added to the Ringer solution used to dialyse the probe, the s.c. administration of both acute and subchronic nicotine (0.4 mg kg-1) resulted in significant increases (P less than 0.05) in the dopamine concentration in the dialysate. Under these conditions, pretreatment with nicotine prior to the test day prolonged (P less than 0.05) the dopamine response to a challenge dose of nicotine.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K., Fuxe K., Agnati L. F. Effects of single injections of nicotine on the ascending dopamine pathways in the rat. Evidence for increases of dopamine turnover in the mesostriatal and mesolimbic dopamine neurons. Acta Physiol Scand. 1981 Jul;112(3):345–347. doi: 10.1111/j.1748-1716.1981.tb06827.x. [DOI] [PubMed] [Google Scholar]
- Andersson K., Fuxe K., Agnati L. F., Eneroth P. Effects of acute central and peripheral administration of nicotine on ascending dopamine pathways in the male rat brain. Evidence for nicotine induced increases of dopamine turnover in various telencephalic dopamine nerve terminal systems. Med Biol. 1981 Jun;59(3):170–176. [PubMed] [Google Scholar]
- Benveniste H. Brain microdialysis. J Neurochem. 1989 Jun;52(6):1667–1679. doi: 10.1111/j.1471-4159.1989.tb07243.x. [DOI] [PubMed] [Google Scholar]
- Clarke P. B., Fu D. S., Jakubovic A., Fibiger H. C. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988 Aug;246(2):701–708. [PubMed] [Google Scholar]
- Clarke P. B., Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983 Feb;78(2):329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R. J., Weeks J. R., Cooper M. M., Good P. I., Russell R. R. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl) 1984;82(1-2):6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Corrigall W. A., Coen K. M. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 1991;104(2):171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Cox B. M., Goldstein A., Nelson W. T. Nicotine self-administration in rats. Br J Pharmacol. 1984 Sep;83(1):49–55. doi: 10.1111/j.1476-5381.1984.tb10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I., Iversen S. D. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975 Jan 17;83(3):419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Damsma G., Day J., Fibiger H. C. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989 Sep 22;168(3):363–368. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. In-vivo brain dialysis of neurotransmitters. Trends Pharmacol Sci. 1990 Mar;11(3):116–121. doi: 10.1016/0165-6147(90)90197-g. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Moore R. Y. Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J Comp Neurol. 1978 Aug 1;180(3):533–544. doi: 10.1002/cne.901800309. [DOI] [PubMed] [Google Scholar]
- Fung Y. K. Effects of chronic nicotine pretreatment on (+)-amphetamine and nicotine-induced synthesis and release of [3H]dopamine from [3H]tyrosine in rat nucleus accumbens. J Pharm Pharmacol. 1989 Jan;41(1):66–68. doi: 10.1111/j.2042-7158.1989.tb06334.x. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Lau Y. S. Receptor mechanisms of nicotine-induced locomotor hyperactivity in chronic nicotine-treated rats. Eur J Pharmacol. 1988 Aug 2;152(3):263–271. doi: 10.1016/0014-2999(88)90721-2. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Andersson K., Härfstrand A., Agnati L. F. Increases in dopamine utilization in certain limbic dopamine terminal populations after a short period of intermittent exposure of male rats to cigarette smoke. J Neural Transm. 1986;67(1-2):15–29. doi: 10.1007/BF01243356. [DOI] [PubMed] [Google Scholar]
- Goldberg S. R., Spealman R. D., Goldberg D. M. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981 Oct 30;214(4520):573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Skirboll L., Rehfeld J. F., Goldstein M., Markey K., Dann O. A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contains a cholecystokinin-like peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience. 1980;5(12):2093–2124. doi: 10.1016/0306-4522(80)90127-x. [DOI] [PubMed] [Google Scholar]
- Imperato A., Mulas A., Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986 Dec 16;132(2-3):337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Izenwasser S., Jacocks H. M., Rosenberger J. G., Cox B. M. Nicotine indirectly inhibits [3H]dopamine uptake at concentrations that do not directly promote [3H]dopamine release in rat striatum. J Neurochem. 1991 Feb;56(2):603–610. doi: 10.1111/j.1471-4159.1991.tb08192.x. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W., Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5(1):48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Kelly P. H., Seviour P. W., Iversen S. D. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975 Sep 5;94(3):507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Lapin E. P., Maker H. S., Sershen H., Hurd Y., Lajtha A. Dopamine-like action of nicotine: lack of tolerance and reverse tolerance. Brain Res. 1987 Mar 31;407(2):351–363. doi: 10.1016/0006-8993(87)91114-0. [DOI] [PubMed] [Google Scholar]
- Lyness W. H., Friedle N. M., Moore K. E. Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav. 1979 Nov;11(5):553–556. doi: 10.1016/0091-3057(79)90040-6. [DOI] [PubMed] [Google Scholar]
- Pettit H. O., Justice J. B., Jr Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991 Jan 18;539(1):94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- Reavill C., Stolerman I. P. Locomotor activity in rats after administration of nicotinic agonists intracerebrally. Br J Pharmacol. 1990 Feb;99(2):273–278. doi: 10.1111/j.1476-5381.1990.tb14693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. E., Whishaw I. Q. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988 May 31;450(1-2):209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Schenk S., Snow S., Horger B. A. Pre-exposure to amphetamine but not nicotine sensitizes rats to the motor activating effect of cocaine. Psychopharmacology (Berl) 1991;103(1):62–66. doi: 10.1007/BF02244075. [DOI] [PubMed] [Google Scholar]
- Singer G., Wallace M., Hall R. Effects of dopaminergic nucleus accumbens lesions on the acquisition of schedule induced self injection of nicotine in the rat. Pharmacol Biochem Behav. 1982 Sep;17(3):579–581. doi: 10.1016/0091-3057(82)90321-5. [DOI] [PubMed] [Google Scholar]
- Stolerman I. P., Fink R., Jarvik M. E. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973 Jun 29;30(4):329–342. doi: 10.1007/BF00429192. [DOI] [PubMed] [Google Scholar]
- Vale A. L., Balfour D. J. Aversive environmental stimuli as a factor in the psychostimulant response to nicotine. Pharmacol Biochem Behav. 1989 Apr;32(4):857–860. doi: 10.1016/0091-3057(89)90048-8. [DOI] [PubMed] [Google Scholar]
- Westerink B. H., De Vries J. B. Characterization of in vivo dopamine release as determined by brain microdialysis after acute and subchronic implantations: methodological aspects. J Neurochem. 1988 Sep;51(3):683–687. doi: 10.1111/j.1471-4159.1988.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Wise R. A., Bozarth M. A. A psychomotor stimulant theory of addiction. Psychol Rev. 1987 Oct;94(4):469–492. [PubMed] [Google Scholar]



