Abstract
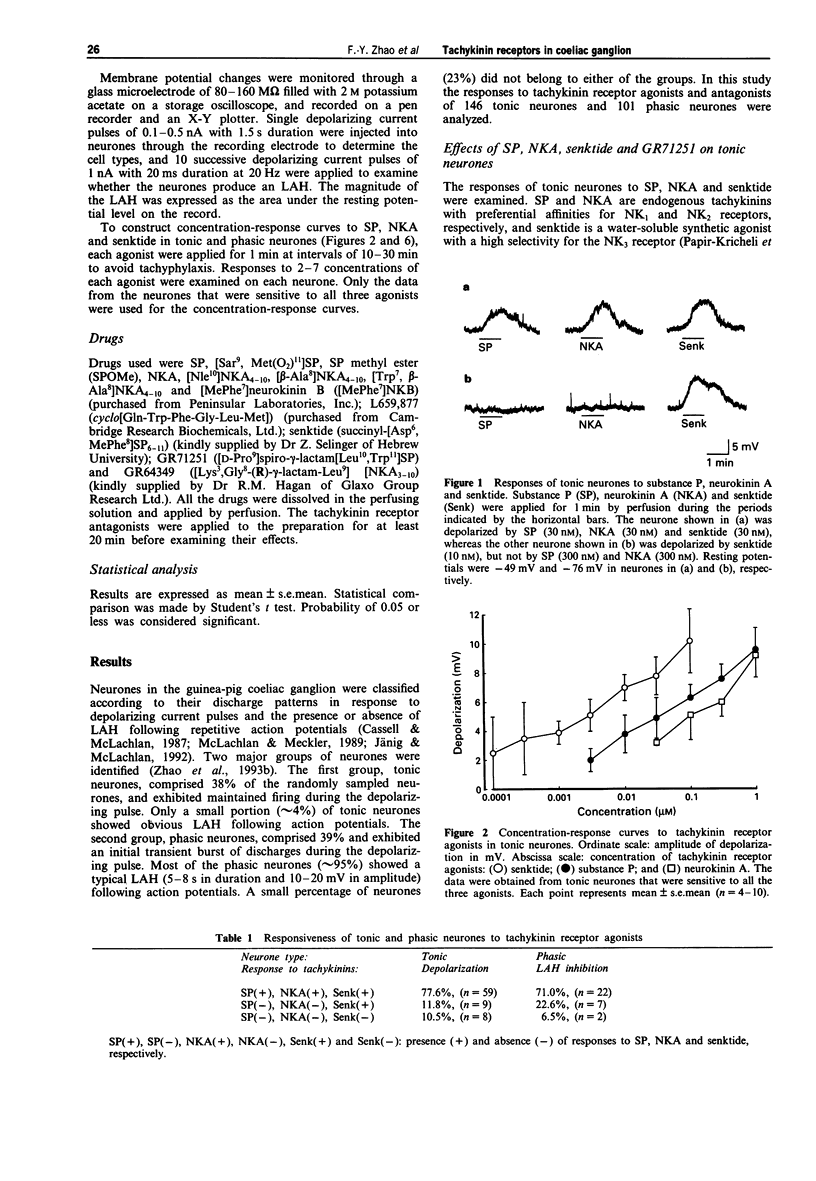
1. Intracellular recording techniques were used to investigate the characteristics of tachykinin receptors and their subtypes in tonic and phasic neurones, which constituted two major neuronal populations in the coeliac ganglion of the guinea-pig. 2. In 95% of phasic neurones a long-lasting after-hyperpolarization (LAH), 5-8 s in duration and 10-20 mV in amplitude, was observed following action potentials evoked by passing a train of depolarizing current pulses into the neurones. In contrast, LAH was observed in only 4% of tonic neurones. 3. In most tonic neurones, substance P (SP), neurokinin A (NKA) and senktide induced depolarizations, whereas in phasic neurones they usually inhibited LAH but rarely induced depolarization. 4. Tonic and phasic neurones were further classified into three groups based on their responses (depolarization for tonic neurones and LAH inhibition for phasic neurones) to these tachykinin receptor agonists: (1) neurones responsive to SP, NKA and senktide (71-78%); (2) those responsive to senktide but not to SP and NKA (12-23%) and (3) those not responsive to any of the three agonists (7-11%). 5. GR71251 (5 microM), an NK1-selective tachykinin receptor antagonist, depressed the depolarization in tonic neurones and the LAH inhibition in phasic neurones induced by SP and NKA, but not those induced by senktide. 6. Selective NK2 receptor agonists, [Nle10]NKA4-10, [beta-Ala8]NKA4-10 and GR64349, were without effect in both tonic and phasic neurones. Furthermore, an NK2 receptor antagonist, L659,877, did not inhibit the depolarization induced by NKA, SP or senktide in tonic neurones.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
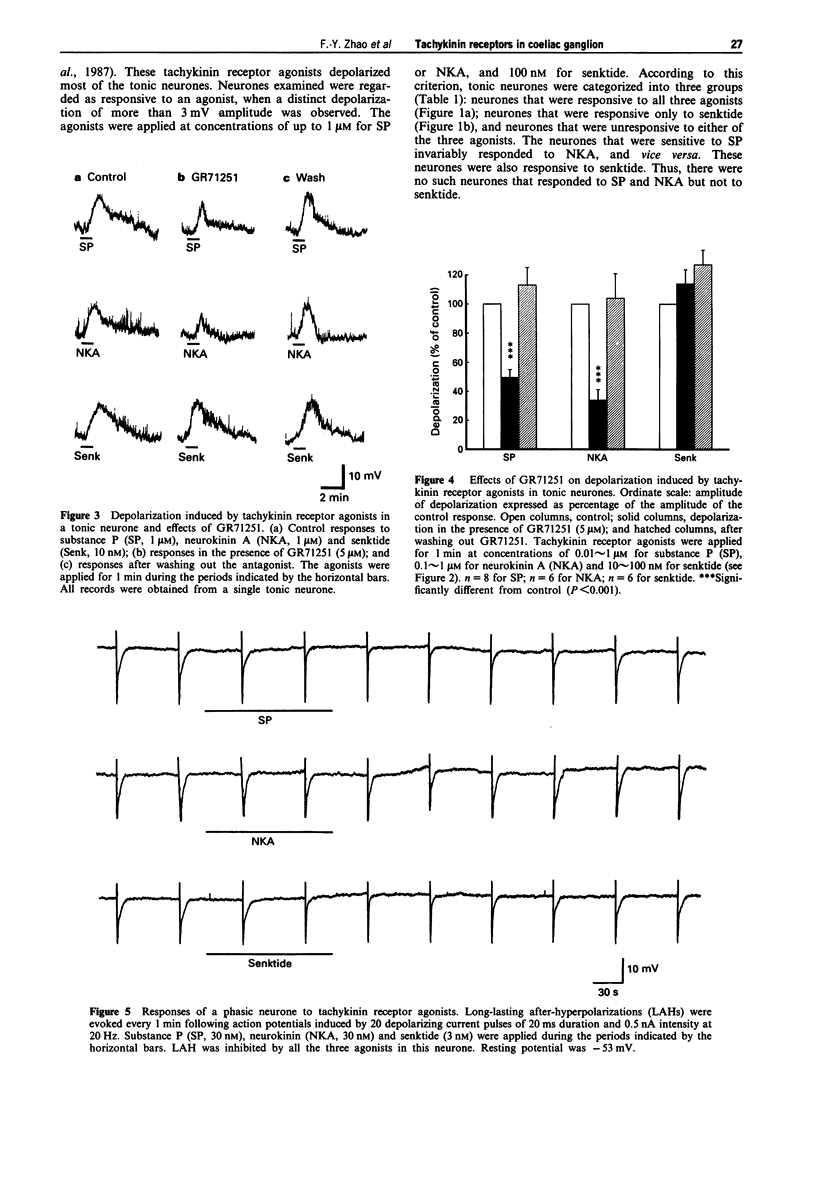

Selected References
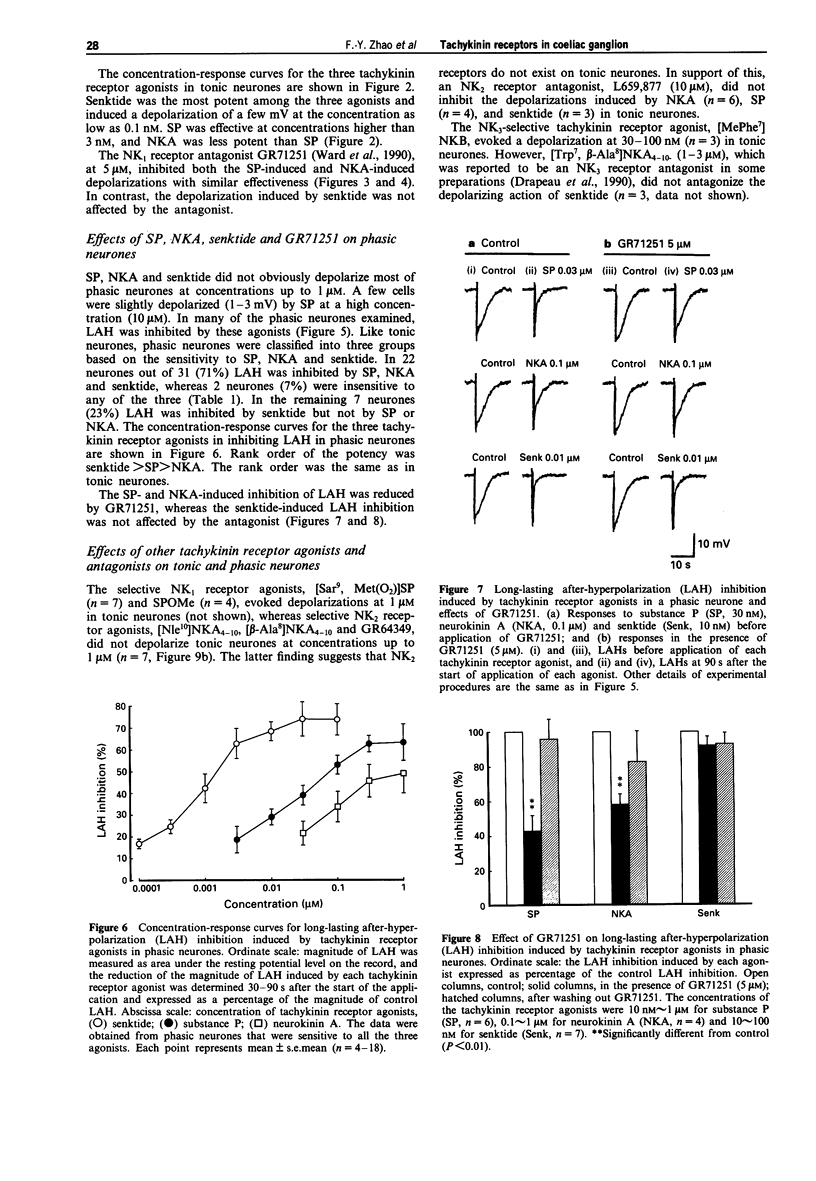
These references are in PubMed. This may not be the complete list of references from this article.
- Cassell J. F., McLachlan E. M. Two calcium-activated potassium conductances in a subpopulation of coeliac neurones of guinea-pig and rabbit. J Physiol. 1987 Dec;394:331–349. doi: 10.1113/jphysiol.1987.sp016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
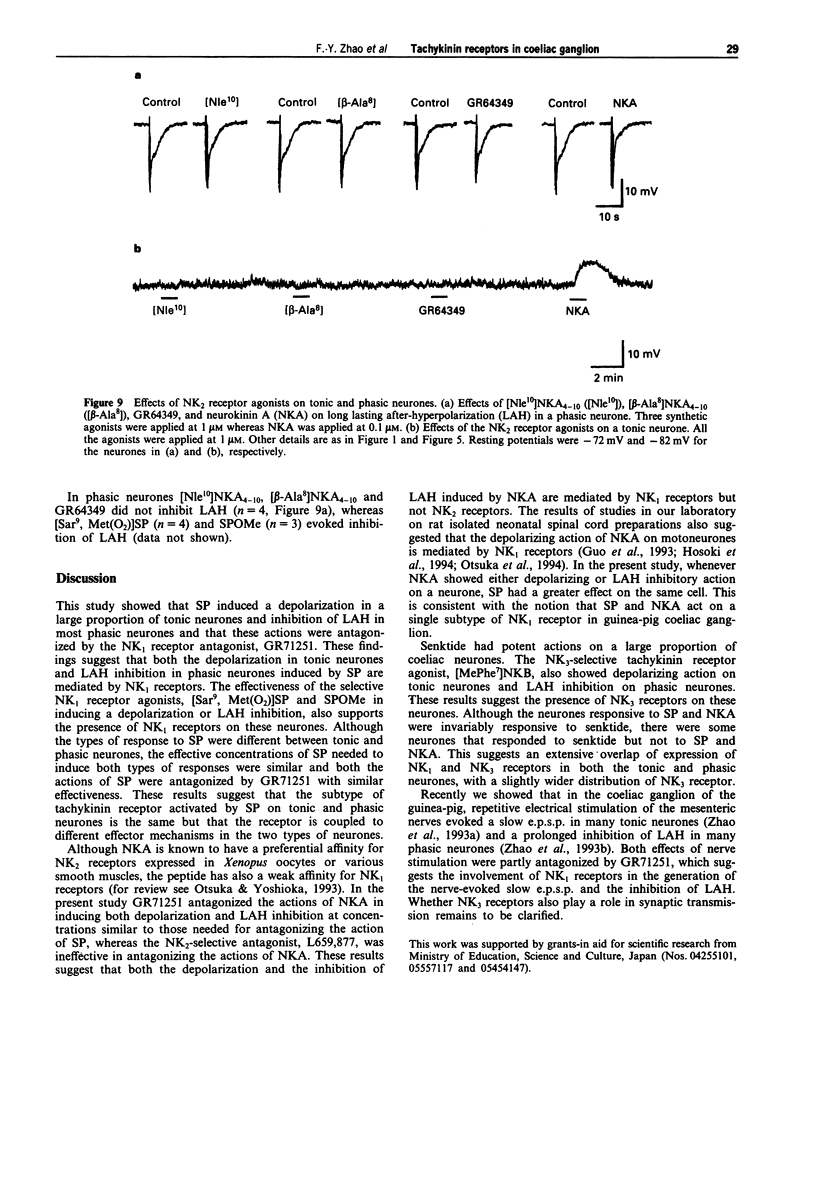
- Drapeau G., Rouissi N., Nantel F., Rhaleb N. E., Tousignant C., Regoli D. Antagonists for the neurokinin NK-3 receptor evaluated in selective receptor systems. Regul Pept. 1990 Nov 15;31(2):125–135. doi: 10.1016/0167-0115(90)90115-d. [DOI] [PubMed] [Google Scholar]
- Guo J. Z., Yoshioka K., Yanagisawa M., Hosoki R., Hagan R. M., Otsuka M. Depression of primary afferent-evoked responses by GR71251 in the isolated spinal cord of the neonatal rat. Br J Pharmacol. 1993 Nov;110(3):1142–1148. doi: 10.1111/j.1476-5381.1993.tb13933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R., Yanagisawa M., Guo J. Z., Yoshioka K., Maehara T., Otsuka M. Effects of RP 67580, a tachykinin NK1 receptor antagonist, on a primary afferent-evoked response of ventral roots in the neonatal rat spinal cord. Br J Pharmacol. 1994 Dec;113(4):1141–1146. doi: 10.1111/j.1476-5381.1994.tb17116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. M. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 1992 Dec;15(12):475–481. doi: 10.1016/0166-2236(92)90092-m. [DOI] [PubMed] [Google Scholar]
- Konishi S., Otsuka M. Blockade of slow excitatory post-synaptic potential by substance P antagonists in guinea-pig sympathetic ganglia. J Physiol. 1985 Apr;361:115–130. doi: 10.1113/jphysiol.1985.sp015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae I. M., Furness J. B., Costa M. Distribution of subgroups of noradrenaline neurons in the coeliac ganglion of the guinea-pig. Cell Tissue Res. 1986;244(1):173–180. doi: 10.1007/BF00218395. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M., Meckler R. L. Characteristics of synaptic input to three classes of sympathetic neurone in the coeliac ganglion of the guinea-pig. J Physiol. 1989 Aug;415:109–129. doi: 10.1113/jphysiol.1989.sp017714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993 Apr;73(2):229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Papir-Kricheli D., Frey J., Laufer R., Gilon C., Chorev M., Selinger Z., Devor M. Behavioural effects of receptor-specific substance P agonists. Pain. 1987 Nov;31(2):263–276. doi: 10.1016/0304-3959(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Saria A., Ma R. C., Dun N. J. Neurokinin A depolarizes neurons of the guinea pig inferior mesenteric ganglia. Neurosci Lett. 1985 Sep 30;60(2):145–150. doi: 10.1016/0304-3940(85)90235-6. [DOI] [PubMed] [Google Scholar]
- Tsunoo A., Konishi S., Otsuka M. Substance P as an excitatory transmitter of primary afferent neurons in guinea-pig sympathetic ganglia. Neuroscience. 1982;7(9):2025–2037. doi: 10.1016/0306-4522(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Vanner S., Evans R. J., Matsumoto S. G., Surprenant A. Potassium currents and their modulation by muscarine and substance P in neuronal cultures from adult guinea pig celiac ganglia. J Neurophysiol. 1993 May;69(5):1632–1644. doi: 10.1152/jn.1993.69.5.1632. [DOI] [PubMed] [Google Scholar]
- Ward P., Ewan G. B., Jordan C. C., Ireland S. J., Hagan R. M., Brown J. R. Potent and highly selective neurokinin antagonists. J Med Chem. 1990 Jul;33(7):1848–1851. doi: 10.1021/jm00169a003. [DOI] [PubMed] [Google Scholar]
- Zhao F. Y., Saito K., Konishi S., Guo J. Z., Murakoshi T., Yoshioka K., Otsuka M. Involvement of NK1 receptors in synaptic transmission in the guinea pig coeliac ganglion. Neurosci Res. 1993 Dec;18(3):245–248. doi: 10.1016/0168-0102(93)90061-t. [DOI] [PubMed] [Google Scholar]



