Abstract
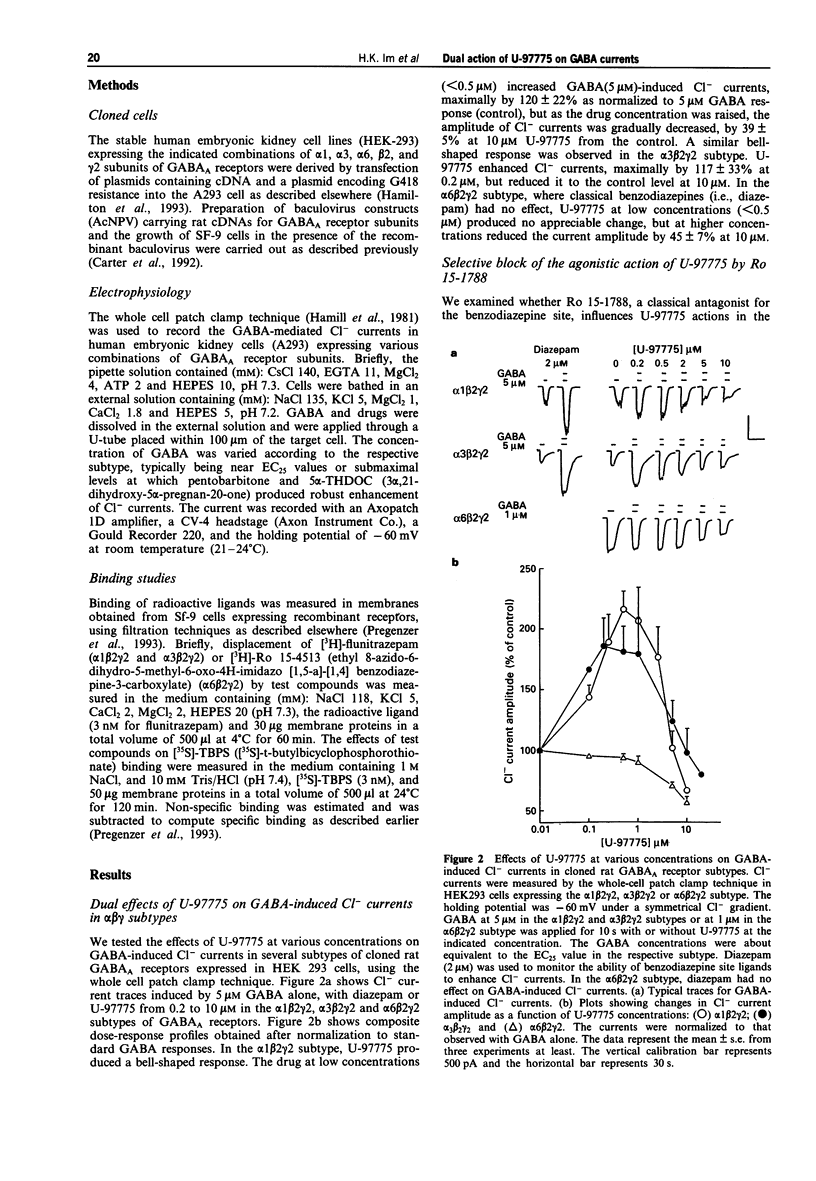
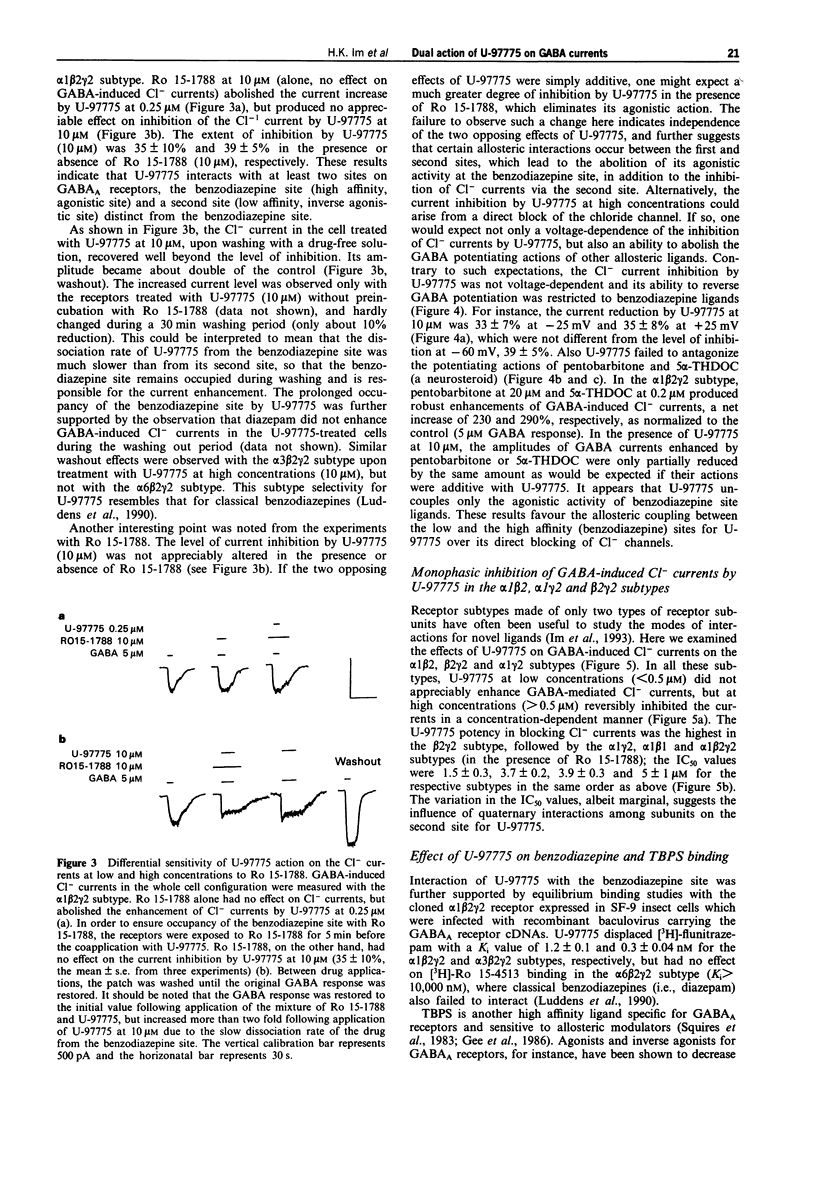
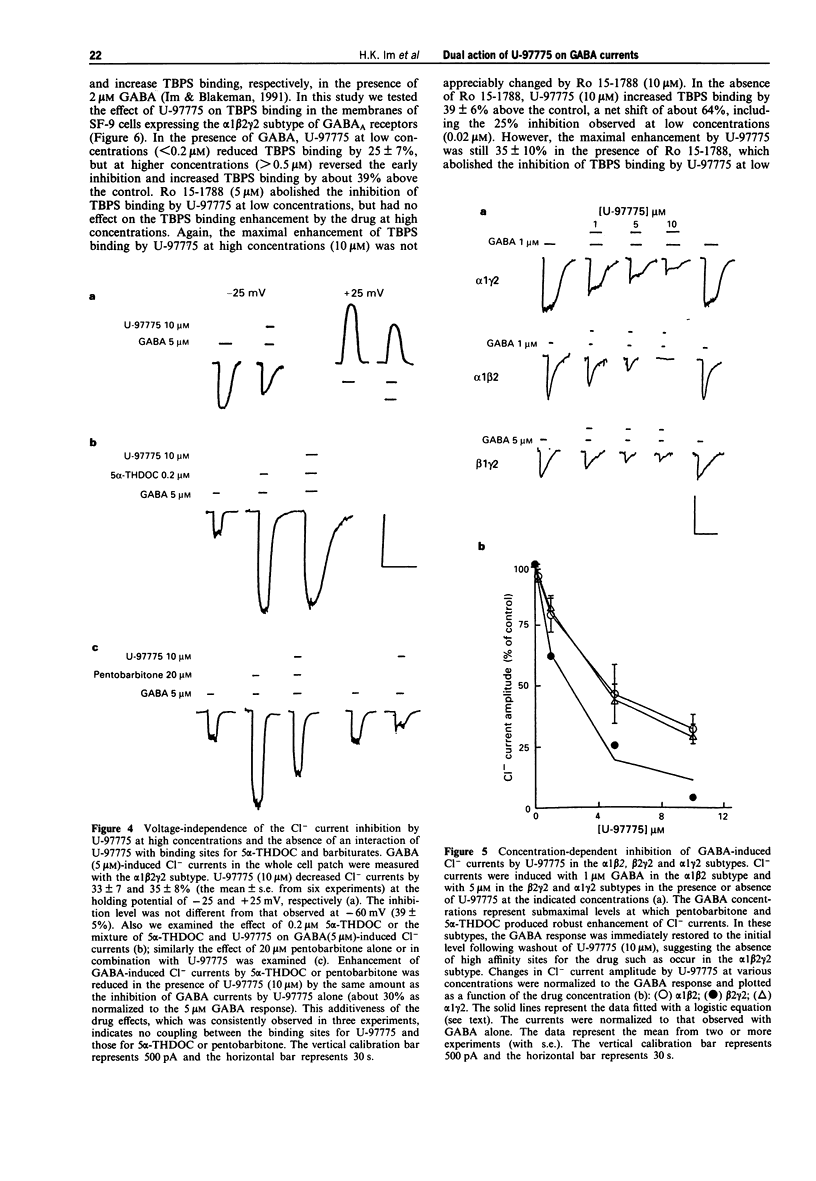
1. U-97775 (tert-butyl 7-chloro-4,5-dihydro-5-[(1-(3,4,5-trimethyl)piperazino)carbonyl]- imidazo[1,5-a])quinoxaline-3-carboxylate) is a novel GABAA receptor ligand of dual functionality and was characterized for its interactions with cloned rat GABAA receptors expressed in human embryonic kidney cells. 2. The drug produced a bell-shaped dose-response profile in the alpha 1 beta 2 gamma 2 receptor subtype as monitored with GABA-induced Cl- currents in the whole cell patch-clamp technique. At low concentrations (< 0.5 microM), U-97775 enhanced the currents with a maximal increase of 120% as normalized to 5 microM GABA response (control). An agonist interaction of U-97775 with the benzodiazepine site is suggested, because Ro 15-1788 (an antagonist at the benzodiazepine site) abolished the current increase and [3H]-flunitrazepam binding was inhibited by U-97775 with a Ki of 1.2 nM. 3. The enhancement of GABA currents progressively disappeared as the U-97775 concentration was raised above 1 microM, and the current amplitude was reduced to 40% below the control at 10 microM U-97775. The current inhibition by U-97775 (10 microM) was not affected by Ro 15-1788. It appears that U-97775 interacts with a second site on GABA receptors, distinct from the benzodiazepine site, to reverse its agonistic activity on the benzodiazepine site and also to inhibit GABA currents. 4. U-97775 at low concentrations reduced and at high concentrations enhanced [35S]-TBPS binding. Ro 15-1788 selectively blocked the effect of U-97775 at low concentrations.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard E. A., Sutherland M., Zaman S., Matsumoto M., Nayeem N., Green T., Darlison M. G., Bateson A. N. Multiplicity, structure, and function in GABAA receptors. Ann N Y Acad Sci. 1993 Dec 20;707:116–125. doi: 10.1111/j.1749-6632.1993.tb38047.x. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Carter D. B., Thomsen D. R., Im W. B., Lennon D. J., Ngo D. M., Gale W., Im H. K., Seeburg P. H., Smith M. W. Functional expression of GABAA chloride channels and benzodiazepine binding sites in baculovirus infected insect cells. Biotechnology (N Y) 1992 Jun;10(6):679–681. doi: 10.1038/nbt0692-679. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Molecular mechanisms in the receptor action of benzodiazepines. Annu Rev Pharmacol Toxicol. 1979;19:531–545. doi: 10.1146/annurev.pa.19.040179.002531. [DOI] [PubMed] [Google Scholar]
- Gee K. W., Lawrence L. J., Yamamura H. I. Modulation of the chloride ionophore by benzodiazepine receptor ligands: influence of gamma-aminobutyric acid and ligand efficacy. Mol Pharmacol. 1986 Sep;30(3):218–225. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton B. J., Lennon D. J., Im H. K., Im W. B., Seeburg P. H., Carter D. B. Stable expression of cloned rat GABAA receptor subunits in a human kidney cell line. Neurosci Lett. 1993 Apr 30;153(2):206–209. doi: 10.1016/0304-3940(93)90323-d. [DOI] [PubMed] [Google Scholar]
- Im H. K., Im W. B., Judge T. M., Gammill R. B., Hamilton B. J., Carter D. B., Pregenzer J. F. Substituted pyrazinones, a new class of allosteric modulators for gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993 Aug;44(2):468–472. [PubMed] [Google Scholar]
- Im W. B., Blakeman D. P. Correlation between gamma-aminobutyric acidA receptor ligand-induced changes in t-butylbicyclophosphoro[35S]thionate binding and 36Cl- uptake in rat cerebrocortical membranes. Mol Pharmacol. 1991 Mar;39(3):394–398. [PubMed] [Google Scholar]
- Lüddens H., Pritchett D. B., Köhler M., Killisch I., Keinänen K., Monyer H., Sprengel R., Seeburg P. H. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990 Aug 16;346(6285):648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Pregenzer J. F., Im W. B., Carter D. B., Thomsen D. R. Comparison of interactions of [3H]muscimol, t-butylbicyclophosphoro[35S]thionate, and [3H]flunitrazepam with cloned gamma-aminobutyric acidA receptors of the alpha 1 beta 2 and alpha 1 beta 2 gamma 2 subtypes. Mol Pharmacol. 1993 May;43(5):801–806. [PubMed] [Google Scholar]
- Schofield P. R. The GABAA receptor: molecular biology reveals a complex picture. Trends Pharmacol Sci. 1989 Dec;10(12):476–478. doi: 10.1016/0165-6147(89)90041-2. [DOI] [PubMed] [Google Scholar]
- Sieghart W. GABAA receptors: ligand-gated Cl- ion channels modulated by multiple drug-binding sites. Trends Pharmacol Sci. 1992 Dec;13(12):446–450. doi: 10.1016/0165-6147(92)90142-s. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Casida J. E., Richardson M., Saederup E. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to gamma-aminobutyric acid-A and ion recognition sites. Mol Pharmacol. 1983 Mar;23(2):326–336. [PubMed] [Google Scholar]



