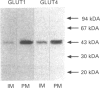
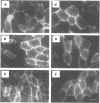
Abstract
1. The present study was designed to clarify the cellular mechanism through which the antihyperglycaemic drug, metformin, exerts its effects. For this purpose the contents of glucose transporter protein isoforms GLUT1 and GLUT4 were measured in plasma membrane and intracellular membrane fractions of skeletal muscle obtained from genetically obese, insulin-resistant Zucker rats. 2. Hindlimb muscles were dissected from metformin-treated (300 mg kg-1 day-1, p.o., for 12 days) and control rats in basal treatment state, and after acute stimulation with insulin (22 u kg-1, i.p.). Since metformin treatment reduces food intake, we also used a pair-fed control group to investigate the effects of altered insulinaemia per se. Glucose transporter levels were analysed by Western blot and slot blot-techniques. In addition, 2-deoxy-[14C]-glucose uptake in isolated muscle strips was evaluated. 3. No changes were noted in the contents of GLUT1 proteins in any of the subcellular fractions after metformin treatment. The contents of GLUT4 in subcellular fractions were not altered in the basal treatment state. After acute insulin exposure the content of GLUT4 in the intracellular membrane fraction declined significantly in the metformin-treated group, while no significant effect was seen in the plasma membrane fraction. In agreement with these results, metformin treatment did not alter 2-deoxyglucose uptake into isolated muscle strips. 4. In conclusion, the present study does not support the concept that metformin would enhance translocation of glucose transporter proteins from the intracellular compartment to the plasma membrane in skeletal muscle in vivo.
Full text
PDF

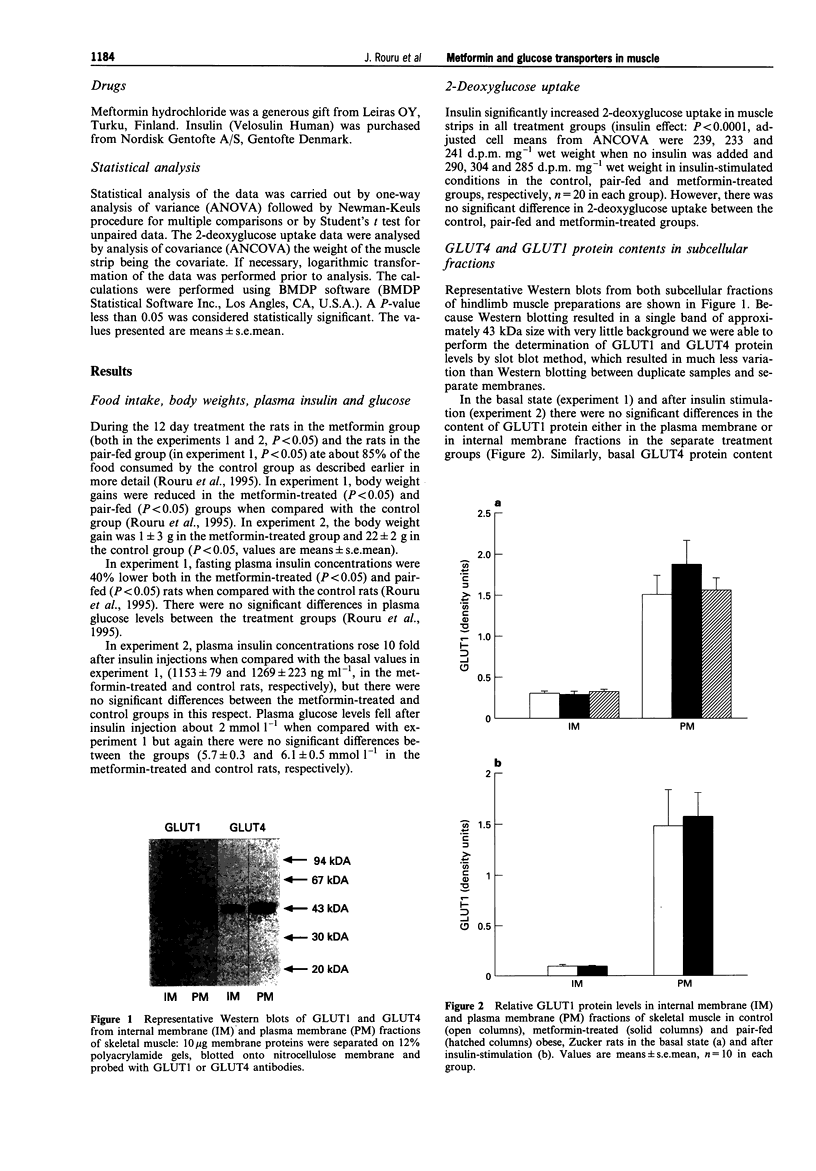
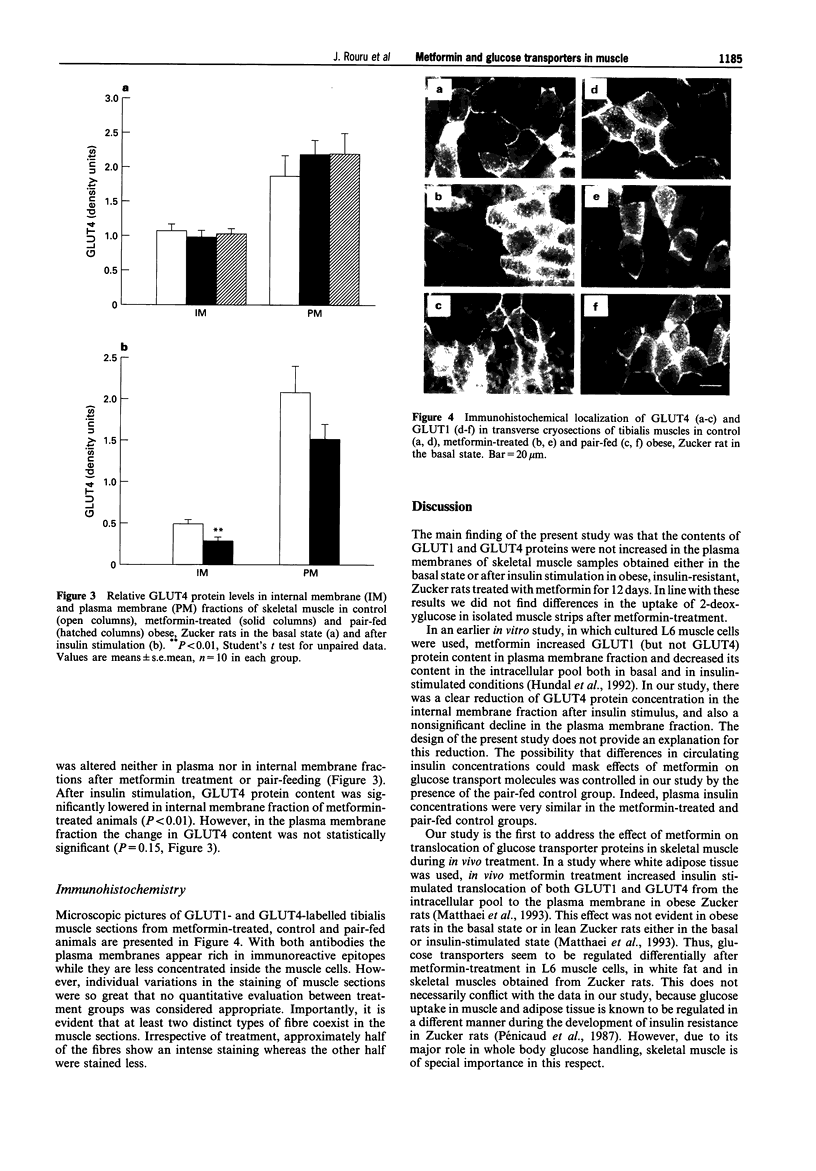


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Bailey C. J. Metformin--an update. Gen Pharmacol. 1993 Nov;24(6):1299–1309. doi: 10.1016/0306-3623(93)90411-p. [DOI] [PubMed] [Google Scholar]
- Bailey C. J., Puah J. A. Effect of metformin on glucose metabolism in mouse soleus muscle. Diabete Metab. 1986 Aug;12(4):212–218. [PubMed] [Google Scholar]
- Bray G. A., York D. A., Fisler J. S. Experimental obesity: a homeostatic failure due to defective nutrient stimulation of the sympathetic nervous system. Vitam Horm. 1989;45:1–125. doi: 10.1016/s0083-6729(08)60393-3. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Prentki M., Zaninetti D., Jeanrenaud B. Insulin resistance in soleus muscle from obese Zucker rats. Involvement of several defective sites. Biochem J. 1980 Feb 15;186(2):525–534. doi: 10.1042/bj1860525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrakoudis D., Vranic M., Klip A. Effects of hyperglycemia on glucose transporters of the muscle: use of the renal glucose reabsorption inhibitor phlorizin to control glycemia. J Am Soc Nephrol. 1992 Nov;3(5):1078–1091. doi: 10.1681/ASN.V351078. [DOI] [PubMed] [Google Scholar]
- Handberg A., Kayser L., Høyer P. E., Micheelsen J., Vinten J. Elevated GLUT 1 level in crude muscle membranes from diabetic Zucker rats despite a normal GLUT 1 level in perineurial sheaths. Diabetologia. 1994 May;37(5):443–448. doi: 10.1007/s001250050130. [DOI] [PubMed] [Google Scholar]
- Handberg A., Kayser L., Høyer P. E., Voldstedlund M., Hansen H. P., Vinten J. Metformin ameliorates diabetes but does not normalize the decreased GLUT 4 content in skeletal muscle of obese (fa/fa) Zucker rats. Diabetologia. 1993 Jun;36(6):481–486. doi: 10.1007/BF02743261. [DOI] [PubMed] [Google Scholar]
- Hundal H. S., Ramlal T., Reyes R., Leiter L. A., Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992 Sep;131(3):1165–1173. doi: 10.1210/endo.131.3.1505458. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Landin K., Tengborn L., Smith U. Metformin and metoprolol CR treatment in non-obese men. J Intern Med. 1994 Apr;235(4):335–341. doi: 10.1111/j.1365-2796.1994.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Landin K., Tengborn L., Smith U. Treating insulin resistance in hypertension with metformin reduces both blood pressure and metabolic risk factors. J Intern Med. 1991 Feb;229(2):181–187. doi: 10.1111/j.1365-2796.1991.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Marette A., Richardson J. M., Ramlal T., Balon T. W., Vranic M., Pessin J. E., Klip A. Abundance, localization, and insulin-induced translocation of glucose transporters in red and white muscle. Am J Physiol. 1992 Aug;263(2 Pt 1):C443–C452. doi: 10.1152/ajpcell.1992.263.2.C443. [DOI] [PubMed] [Google Scholar]
- Matthaei S., Reibold J. P., Hamann A., Benecke H., Häring H. U., Greten H., Klein H. H. In vivo metformin treatment ameliorates insulin resistance: evidence for potentiation of insulin-induced translocation and increased functional activity of glucose transporters in obese (fa/fa) Zucker rat adipocytes. Endocrinology. 1993 Jul;133(1):304–311. doi: 10.1210/endo.133.1.8391425. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994 Feb 1;219(3):713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Muona P., Sollberg S., Peltonen J., Uitto J. Glucose transporters of rat peripheral nerve. Differential expression of GLUT1 gene by Schwann cells and perineural cells in vivo and in vitro. Diabetes. 1992 Dec;41(12):1587–1596. doi: 10.2337/diab.41.12.1587. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pénicaud L., Ferré P., Terretaz J., Kinebanyan M. F., Leturque A., Doré E., Girard J., Jeanrenaud B., Picon L. Development of obesity in Zucker rats. Early insulin resistance in muscles but normal sensitivity in white adipose tissue. Diabetes. 1987 May;36(5):626–631. doi: 10.2337/diab.36.5.626. [DOI] [PubMed] [Google Scholar]
- Rossetti L., DeFronzo R. A., Gherzi R., Stein P., Andraghetti G., Falzetti G., Shulman G. I., Klein-Robbenhaar E., Cordera R. Effect of metformin treatment on insulin action in diabetic rats: in vivo and in vitro correlations. Metabolism. 1990 Apr;39(4):425–435. doi: 10.1016/0026-0495(90)90259-f. [DOI] [PubMed] [Google Scholar]
- Rouru J., Huupponen R., Santti E., Koulu M. Effect of subchronic metformin treatment on macronutrient selection in genetically obese Zucker rats. Pharmacol Toxicol. 1993 Apr-May;72(4-5):300–303. doi: 10.1111/j.1600-0773.1993.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Rouru J., Pesonen U., Koulu M., Huupponen R., Santti E., Virtanen K., Rouvari T., Jhanwar-Uniyal M. Anorectic effect of metformin in obese Zucker rats: lack of evidence for the involvement of neuropeptide Y. Eur J Pharmacol. 1995 Jan 24;273(1-2):99–106. doi: 10.1016/0014-2999(94)00669-x. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H. Action of insulin on glucose metabolism in vivo. Baillieres Clin Endocrinol Metab. 1993 Oct;7(4):903–927. doi: 10.1016/s0950-351x(05)80239-3. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H. Pathogenesis of non-insulin-dependent diabetes mellitus. Lancet. 1994 Jan 8;343(8889):91–95. doi: 10.1016/s0140-6736(94)90821-4. [DOI] [PubMed] [Google Scholar]