Abstract
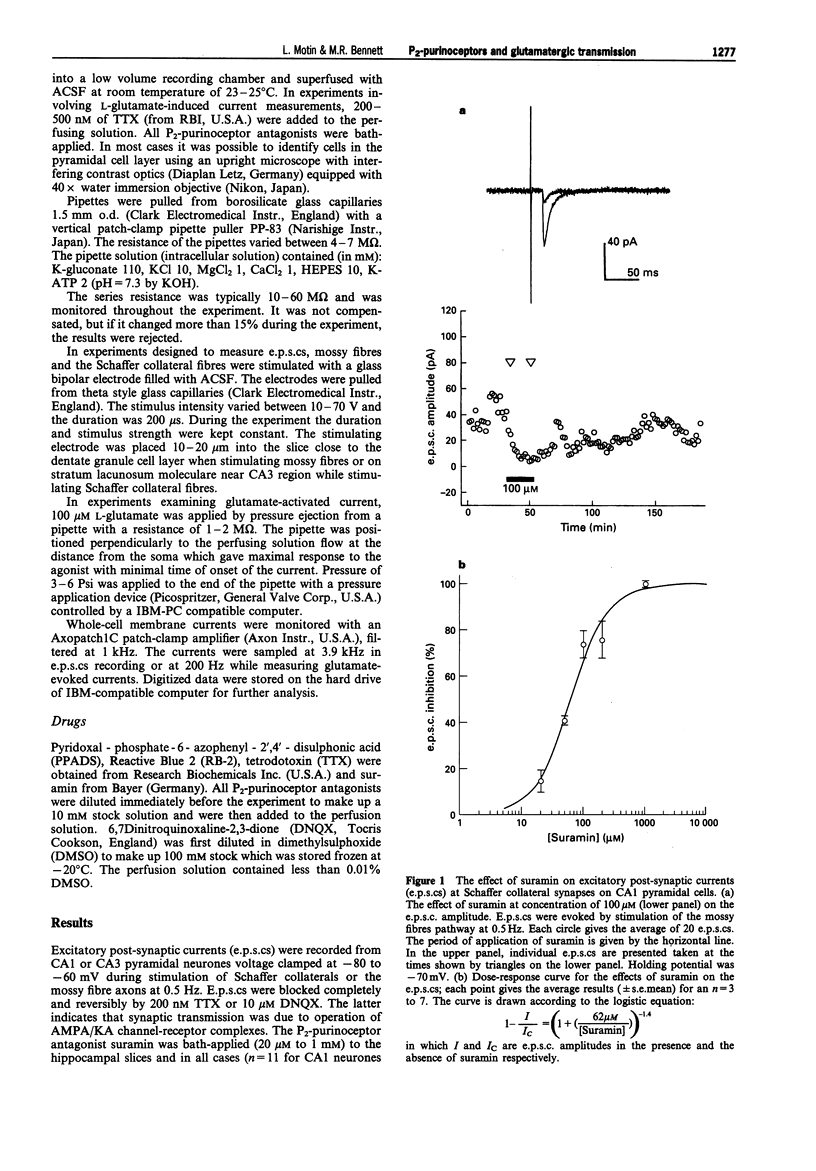
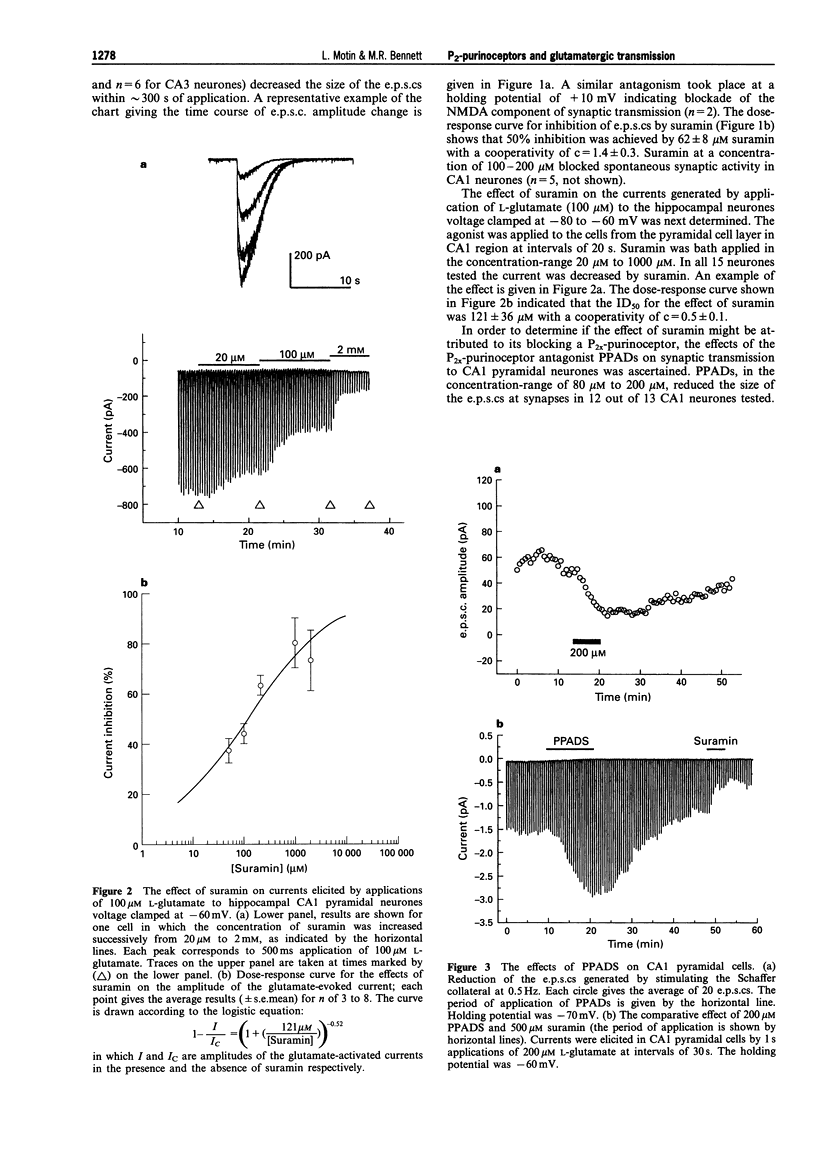
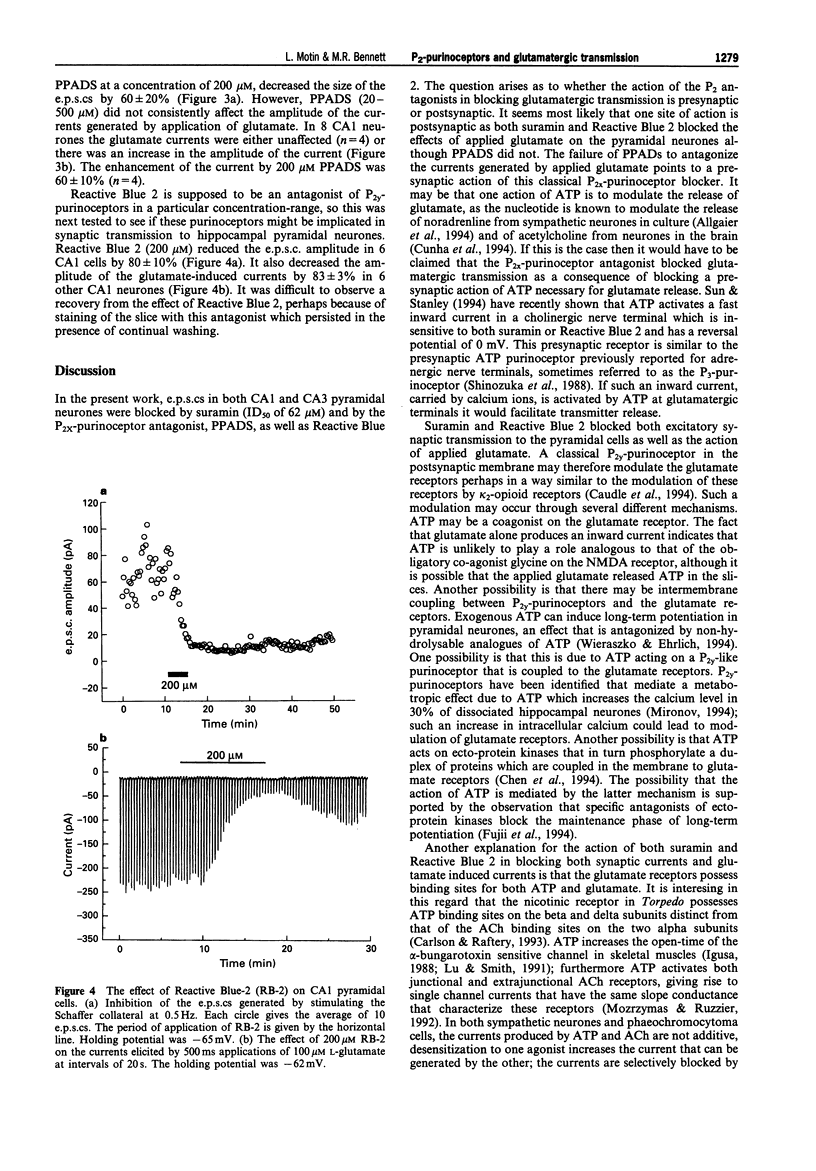
1. A study has been made of the effects of P2-purinoceptor antagonists on the evoked excitatory postsynaptic currents (e.p.s.cs) generated in CA1 pyramidal cells on stimulation of Schaffer collaterals and in CA3 pyramidal cells on stimulation of mossy fibres. The effects of these antagonists on currents generated in the cells on application of glutamate has also been determined. 2. Suramin blocked the evoked e.p.s.cs with an 50% inhibition (ID50) of 62 +/- 8 microM (mean +/- s.e.mean, n = 17), spontaneous miniature e.p.s.cs and the currents induced by application of 100 microM glutamate with an ID50 = 121 +/- 36 microM (n = 15) in all the cells studied. 3. Reactive Blue 2 (RB-2) in a concentration of 200 microM decreased the e.p.s.cs by 80 +/- 10% (n = 6) and the glutamate-activated currents by 83 +/- 3% (n = 6). 4. Pyridoxal-phosphate-6-azophenyl-2',4'-disulphonic acid (PPADS) in the concentration-range of 40-500 microM decreased the amplitude of the e.p.s.cs in 12 out of 13 cells studied. PPADS at 200 microM reduced the amplitude of the e.p.s.cs by 60 +/- 10% (n = 3). PPADS did not affect the glutamate-induced currents in 4 cells and produced potentiation of the current amplitude by 60 +/- 10% in 4 other cells. 5. These results suggest that both presynaptic and postsynaptic P2-purinoceptors in the hippocampus can modulate the release and action of endogenous glutamate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bo X., Burnstock G. Distribution of [3H]alpha,beta-methylene ATP binding sites in rat brain and spinal cord. Neuroreport. 1994 Aug 15;5(13):1601–1604. doi: 10.1097/00001756-199408150-00015. [DOI] [PubMed] [Google Scholar]
- Bobker D. H., Williams J. T. Ion conductances affected by 5-HT receptor subtypes in mammalian neurons. Trends Neurosci. 1990 May;13(5):169–173. doi: 10.1016/0166-2236(90)90042-9. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Dual control of local blood flow by purines. Ann N Y Acad Sci. 1990;603:31–45. doi: 10.1111/j.1749-6632.1990.tb37659.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Carlson B. J., Raftery M. A. Specific binding of ATP to extracellular sites on Torpedo acetylcholine receptor. Biochemistry. 1993 Jul 27;32(29):7329–7333. doi: 10.1021/bi00080a002. [DOI] [PubMed] [Google Scholar]
- Caudle R. M., Chavkin C., Dubner R. Kappa 2 opioid receptors inhibit NMDA receptor-mediated synaptic currents in guinea pig CA3 pyramidal cells. J Neurosci. 1994 Sep;14(9):5580–5589. doi: 10.1523/JNEUROSCI.14-09-05580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha R. A., Ribeiro J. A., Sebastião A. M. Purinergic modulation of the evoked release of [3H]acetylcholine from the hippocampus and cerebral cortex of the rat: role of the ectonucleotidases. Eur J Neurosci. 1994 Jan 1;6(1):33–42. doi: 10.1111/j.1460-9568.1994.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Grassi F., Polenzani L., Mileo A. M., Caratsch C. G., Eusebi F., Miledi R. Blockage of nicotinic acetylcholine receptors by 5-hydroxytryptamine. J Neurosci Res. 1993 Apr 1;34(5):562–570. doi: 10.1002/jnr.490340508. [DOI] [PubMed] [Google Scholar]
- Headley P. M., Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol Sci. 1990 May;11(5):205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- Igusa Y. Adenosine 5'-triphosphate activates acetylcholine receptor channels in cultured Xenopus myotomal muscle cells. J Physiol. 1988 Nov;405:169–185. doi: 10.1113/jphysiol.1988.sp017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Watano T., Ohara-Imaizumi M., Fujimori K., Takanaka A. Dopamine receptor agonists and antagonists enhance ATP-activated currents. Eur J Pharmacol. 1992 May 14;215(2-3):321–324. doi: 10.1016/0014-2999(92)90049-a. [DOI] [PubMed] [Google Scholar]
- Lu Z., Smith D. O. Adenosine 5'-triphosphate increases acetylcholine channel opening frequency in rat skeletal muscle. J Physiol. 1991 May;436:45–56. doi: 10.1113/jphysiol.1991.sp018538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A. D., Humphrey P. P. Distribution and characterisation of [3H]alpha,beta-methylene ATP binding sites in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1993 Dec;348(6):608–617. doi: 10.1007/BF00167237. [DOI] [PubMed] [Google Scholar]
- Mironov S. L. Metabotropic ATP receptor in hippocampal and thalamic neurones: pharmacology and modulation of Ca2+ mobilizing mechanisms. Neuropharmacology. 1994 Jan;33(1):1–13. doi: 10.1016/0028-3908(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Mozrzymas J. W., Ruzzier F. ATP activates junctional and extrajunctional acetylcholine receptor channels in isolated adult rat muscle fibres. Neurosci Lett. 1992 May 25;139(2):217–220. doi: 10.1016/0304-3940(92)90556-m. [DOI] [PubMed] [Google Scholar]
- Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J Neurosci. 1994 Feb;14(2):740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S., Mohri M., Okada Y., Mori M. Excitatory and inhibitory effects of adenosine on the neurotransmission in the hippocampal slices of guinea pig. Brain Res. 1990 Aug 13;525(1):165–169. doi: 10.1016/0006-8993(90)91335-e. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nishimura S., Miyamoto T. Excitatory effect of adenosine on neurotransmission in the slices of superior colliculus and hippocampus of guinea pig. Neurosci Lett. 1990 Dec 11;120(2):205–208. doi: 10.1016/0304-3940(90)90039-c. [DOI] [PubMed] [Google Scholar]
- Shinozuka K., Bjur R. A., Westfall D. P. Inhibition of norepinephrine release from vascular adrenergic nerves by adenyl purines. Proc West Pharmacol Soc. 1988;31:193–195. [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J Physiol. 1993 May;464:197–212. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V., Vanner S. M. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992 Aug;106(4):762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W., Cusack N. J. Absence of P2-purinoceptors in hippocampal pathways. Br J Pharmacol. 1989 Jun;97(2):631–635. doi: 10.1111/j.1476-5381.1989.tb11996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieraszko A., Ehrlich Y. H. On the role of extracellular ATP in the induction of long-term potentiation in the hippocampus. J Neurochem. 1994 Nov;63(5):1731–1738. doi: 10.1046/j.1471-4159.1994.63051731.x. [DOI] [PubMed] [Google Scholar]
- Wieraszko A., Goldsmith G., Seyfried T. N. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 1989 Apr 24;485(2):244–250. doi: 10.1016/0006-8993(89)90567-2. [DOI] [PubMed] [Google Scholar]
- Wieraszko A., Seyfried T. N. ATP-induced synaptic potentiation in hippocampal slices. Brain Res. 1989 Jul 10;491(2):356–359. doi: 10.1016/0006-8993(89)90070-x. [DOI] [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W. Distribution and release of adenosine triphosphate in rat brain. Neurochem Res. 1978 Oct;3(5):563–571. doi: 10.1007/BF00963759. [DOI] [PubMed] [Google Scholar]


