Abstract
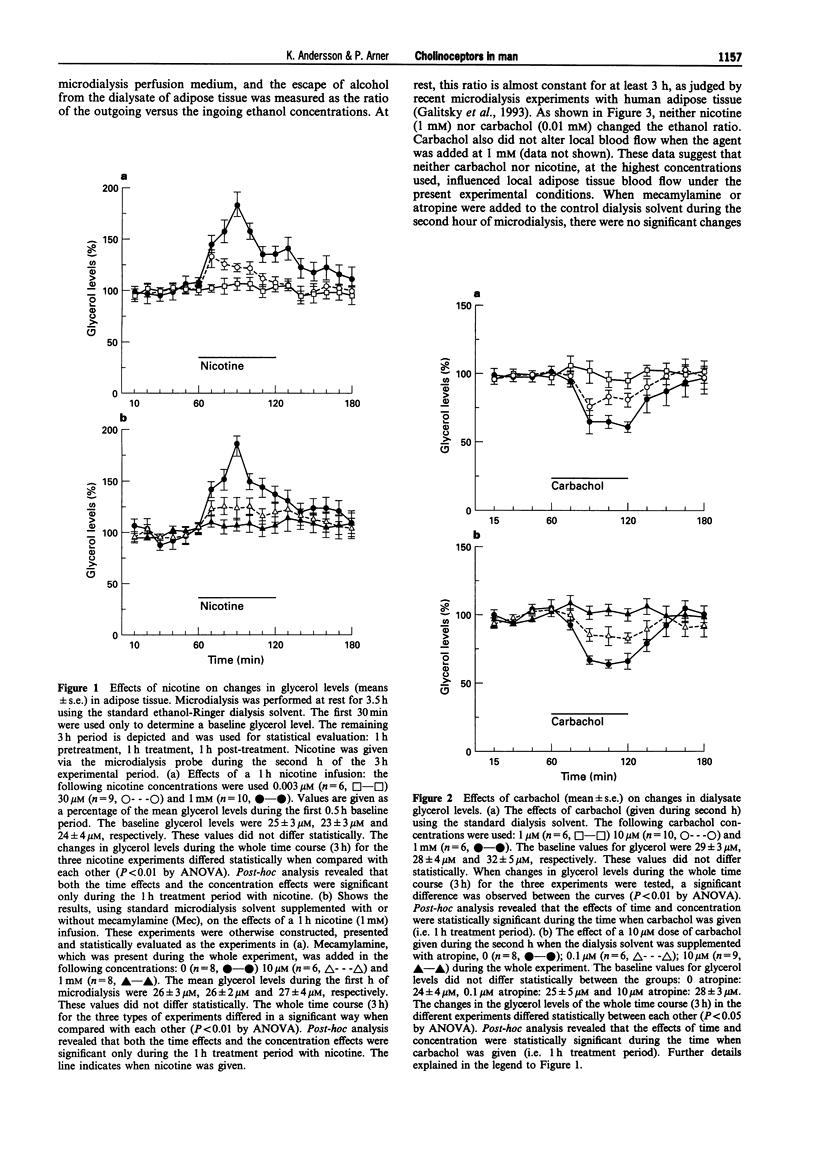
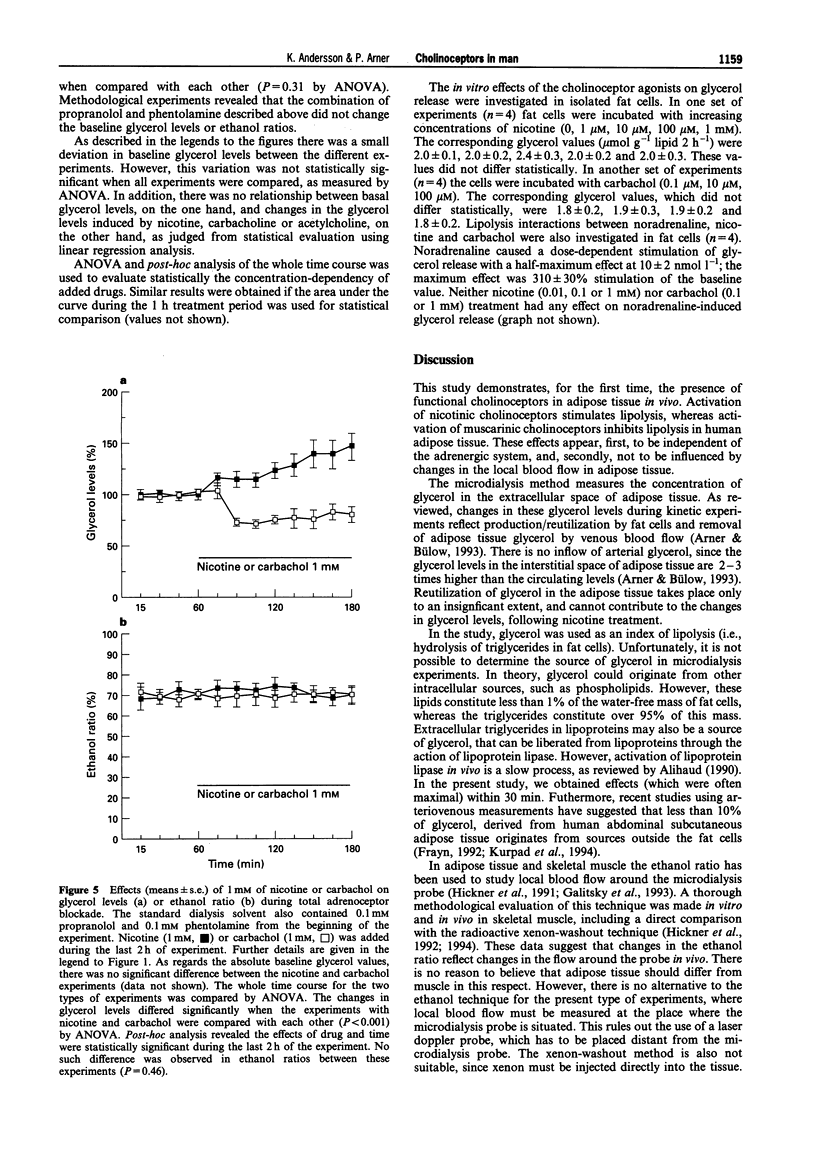
1. Possible cholinoceptor-mediated effects on lipolysis were investigated in vivo in human subcutaneous adipose tissue of non-obese, non-smoking, healthy subjects, by use of microdialysis. Cholinomimetic and sympathomimetic agents were added to the ingoing dialysate solvent. 2. Addition of nicotine to the perfusion solvent caused a concentration-dependent reversible increase in the levels of glycerol in the dialysate (lipolysis index). The opposite effect (also concentration-dependent and reversible) was caused by the addition of carbachol. The maximum effects were 100% stimulation and 50% inhibition, respectively, by nicotine and carbachol. Neither nicotine nor carbachol stimulated nutritive blood flow in adipose tissue (as measured with an ethanol escape technique). 3. The nicotine effect in situ was concentration-dependently counteracted by the nicotinic cholinoceptor antagonist, mecamylamine. Likewise, the carbachol effect was concentration-dependently counteracted by the muscarinic cholinoceptor antagonist, atropine. 4. When adipose tissue was pretreated with phentolamine plus propranolol in order to obtain a complete alpha and beta-adrenoceptor blockade, the subsequent addition of nicotine or carbachol still induced an increase and decrease in dialysate glycerol levels (lipolytic or antilipolytic effects), respectively. When adipose tissue was pretreated with mecamylamine or atropine, the subsequent addition of acetylcholine caused a reversible decrease and increase, respectively, of the dialysate glycerol levels. 5. Nicotine and carbachol had no effects on glycerol release from human isolated subcutaneous fat cells that were incubated in vivo.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ailhaud G. Cellular and secreted lipoprotein lipase revisited. Clin Biochem. 1990 Oct;23(5):343–347. doi: 10.1016/0009-9120(90)90034-r. [DOI] [PubMed] [Google Scholar]
- Anderson K., Fuxe K., Eneroth P., Agnati L. F. Differential effects of mecamylamine on the nicotine induced changes in amine levels and turnover in hypothalamic dopamine and noradrenaline nerve terminal systems and in the secretion of adenohypophyseal hormones in the castrated female rat. Evidence for involvement of cholinergic nicotine-like receptors. Acta Physiol Scand. 1984 Apr;120(4):489–498. doi: 10.1111/j.1748-1716.1984.tb07412.x. [DOI] [PubMed] [Google Scholar]
- Andersson K. Mecamylamine pretreatment counteracts cigarette smoke induced changes in hypothalamic catecholamine neuron systems and in anterior pituitary function. Acta Physiol Scand. 1985 Nov;125(3):445–452. doi: 10.1111/j.1748-1716.1985.tb07741.x. [DOI] [PubMed] [Google Scholar]
- Arner P. Adrenergic receptor function in fat cells. Am J Clin Nutr. 1992 Jan;55(1 Suppl):228S–236S. doi: 10.1093/ajcn/55.1.228s. [DOI] [PubMed] [Google Scholar]
- Arner P., Bülow J. Assessment of adipose tissue metabolism in man: comparison of Fick and microdialysis techniques. Clin Sci (Lond) 1993 Sep;85(3):247–256. doi: 10.1042/cs0850247. [DOI] [PubMed] [Google Scholar]
- Arner P., Hellmér J., Hagström-Toft E., Bolinder J. Effect of phosphodiesterase inhibition with amrinone or theophylline on lipolysis and blood flow in human adipose tissue in vivo as measured with microdialysis. J Lipid Res. 1993 Oct;34(10):1737–1743. [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P., Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest. 1990 Mar;85(3):893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P. In vivo interactions between beta-1 and beta-2 adrenoceptors regulate catecholamine tachyphylaxia in human adipose tissue. J Pharmacol Exp Ther. 1991 Oct;259(1):317–322. [PubMed] [Google Scholar]
- Benveniste H., Hansen A. J., Ottosen N. S. Determination of brain interstitial concentrations by microdialysis. J Neurochem. 1989 Jun;52(6):1741–1750. doi: 10.1111/j.1471-4159.1989.tb07252.x. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Klimkowski V. J., Ellis J. Structure/function relationships of muscarinic acetylcholine receptors. Life Sci. 1993;52(5-6):405–412. doi: 10.1016/0024-3205(93)90295-e. [DOI] [PubMed] [Google Scholar]
- Bungay P. M., Morrison P. F., Dedrick R. L. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46(2):105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- Chajek-Shaul T., Scherer G., Barash V., Shiloni E., Caine Y., Stein O., Stein Y. Metabolic effects of nicotine on human adipose tissue in organ culture. Clin Investig. 1994 Jan;72(2):94–99. doi: 10.1007/BF00184583. [DOI] [PubMed] [Google Scholar]
- Deneris E. S., Connolly J., Rogers S. W., Duvoisin R. Pharmacological and functional diversity of neuronal nicotinic acetylcholine receptors. Trends Pharmacol Sci. 1991 Jan;12(1):34–40. doi: 10.1016/0165-6147(91)90486-c. [DOI] [PubMed] [Google Scholar]
- Enocksson S., Shimizu M., Lönnqvist F., Nordenström J., Arner P. Demonstration of an in vivo functional beta 3-adrenoceptor in man. J Clin Invest. 1995 May;95(5):2239–2245. doi: 10.1172/JCI117914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S. B., Harley E. A., Iversen L. L. Biochemical measurement of muscarinic receptor efficacy and its role in receptor regulation. Trends Pharmacol Sci. 1988 Feb;Suppl:54–60. [PubMed] [Google Scholar]
- Galitzky J., Lafontan M., Nordenström J., Arner P. Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. J Clin Invest. 1993 May;91(5):1997–2003. doi: 10.1172/JCI116421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B., Blank E., Dodds W. J. Nicotine stimulates esophageal peristaltic contractions in cats by a central mechanism. Am J Physiol. 1992 Mar;262(3 Pt 1):G567–G571. doi: 10.1152/ajpgi.1992.262.3.G567. [DOI] [PubMed] [Google Scholar]
- Grenhoff J., Svensson T. H. Pharmacology of nicotine. Br J Addict. 1989 May;84(5):477–492. doi: 10.1111/j.1360-0443.1989.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Hellmér J., Arner P., Lundin A. Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal Biochem. 1989 Feb 15;177(1):132–137. doi: 10.1016/0003-2697(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Bone D., Ungerstedt U., Jorfeldt L., Henriksson J. Muscle blood flow during intermittent exercise: comparison of the microdialysis ethanol technique and 133Xe clearance. Clin Sci (Lond) 1994 Jan;86(1):15–25. doi: 10.1042/cs0860015. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Rosdahl H., Borg I., Ungerstedt U., Jorfeldt L., Henriksson J. Ethanol may be used with the microdialysis technique to monitor blood flow changes in skeletal muscle: dialysate glucose concentration is blood-flow-dependent. Acta Physiol Scand. 1991 Nov;143(3):355–356. doi: 10.1111/j.1748-1716.1991.tb09243.x. [DOI] [PubMed] [Google Scholar]
- Hickner R. C., Rosdahl H., Borg I., Ungerstedt U., Jorfeldt L., Henriksson J. The ethanol technique of monitoring local blood flow changes in rat skeletal muscle: implications for microdialysis. Acta Physiol Scand. 1992 Sep;146(1):87–97. doi: 10.1111/j.1748-1716.1992.tb09396.x. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Fried S. K., Edens N. K., Leibel R. L. The fat cell. Med Clin North Am. 1989 Jan;73(1):83–96. doi: 10.1016/s0025-7125(16)30693-9. [DOI] [PubMed] [Google Scholar]
- Kurpad A., Khan K., Calder A. G., Coppack S., Frayn K., Macdonald I., Elia M. Effect of noradrenaline on glycerol turnover and lipolysis in the whole body and subcutaneous adipose tissue in humans in vivo. Clin Sci (Lond) 1994 Feb;86(2):177–184. doi: 10.1042/cs0860177. [DOI] [PubMed] [Google Scholar]
- Lindh B., Hökfelt T. Structural and functional aspects of acetylcholine peptide coexistence in the autonomic nervous system. Prog Brain Res. 1990;84:175–191. doi: 10.1016/s0079-6123(08)60902-4. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F., Wahrenberg H., Hellström L., Reynisdottir S., Arner P. Lipolytic catecholamine resistance due to decreased beta 2-adrenoceptor expression in fat cells. J Clin Invest. 1992 Dec;90(6):2175–2186. doi: 10.1172/JCI116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. N., Altman D. G., Campbell M. J., Royston P. Analysis of serial measurements in medical research. BMJ. 1990 Jan 27;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride P. E. The health consequences of smoking. Cardiovascular diseases. Med Clin North Am. 1992 Mar;76(2):333–353. doi: 10.1016/s0025-7125(16)30356-x. [DOI] [PubMed] [Google Scholar]
- Niroomand F., Bangert M., Philipps C., Rauch B. Muscarinic receptor-mediated inhibition of GDP-activated adenylyl cyclase suggests a direct interaction of inhibitory guanine nucleotide-binding proteins and adenylyl cyclase. Mol Pharmacol. 1993 Jan;43(1):90–95. [PubMed] [Google Scholar]
- Rosell S., Belfrage E. Blood circulation in adipose tissue. Physiol Rev. 1979 Oct;59(4):1078–1104. doi: 10.1152/physrev.1979.59.4.1078. [DOI] [PubMed] [Google Scholar]
- Tossman U., Ungerstedt U. Microdialysis in the study of extracellular levels of amino acids in the rat brain. Acta Physiol Scand. 1986 Sep;128(1):9–14. doi: 10.1111/j.1748-1716.1986.tb07943.x. [DOI] [PubMed] [Google Scholar]


