Abstract
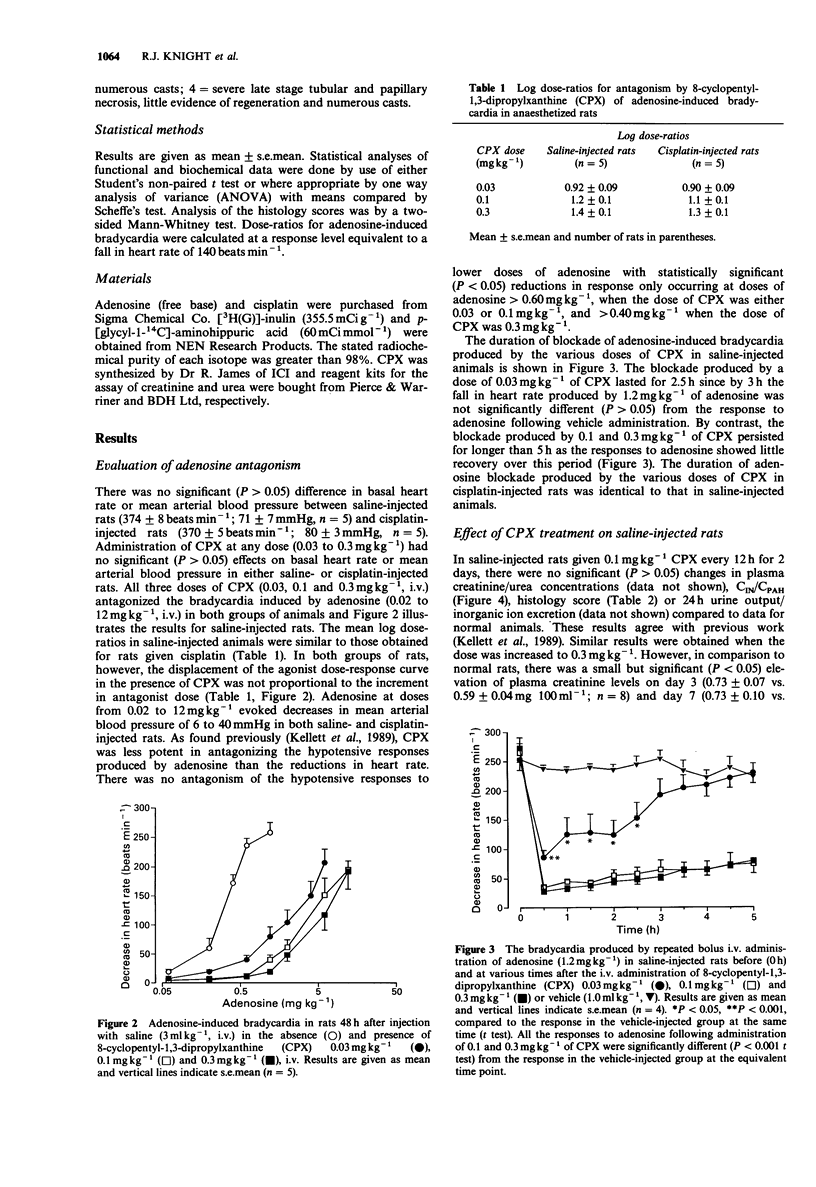
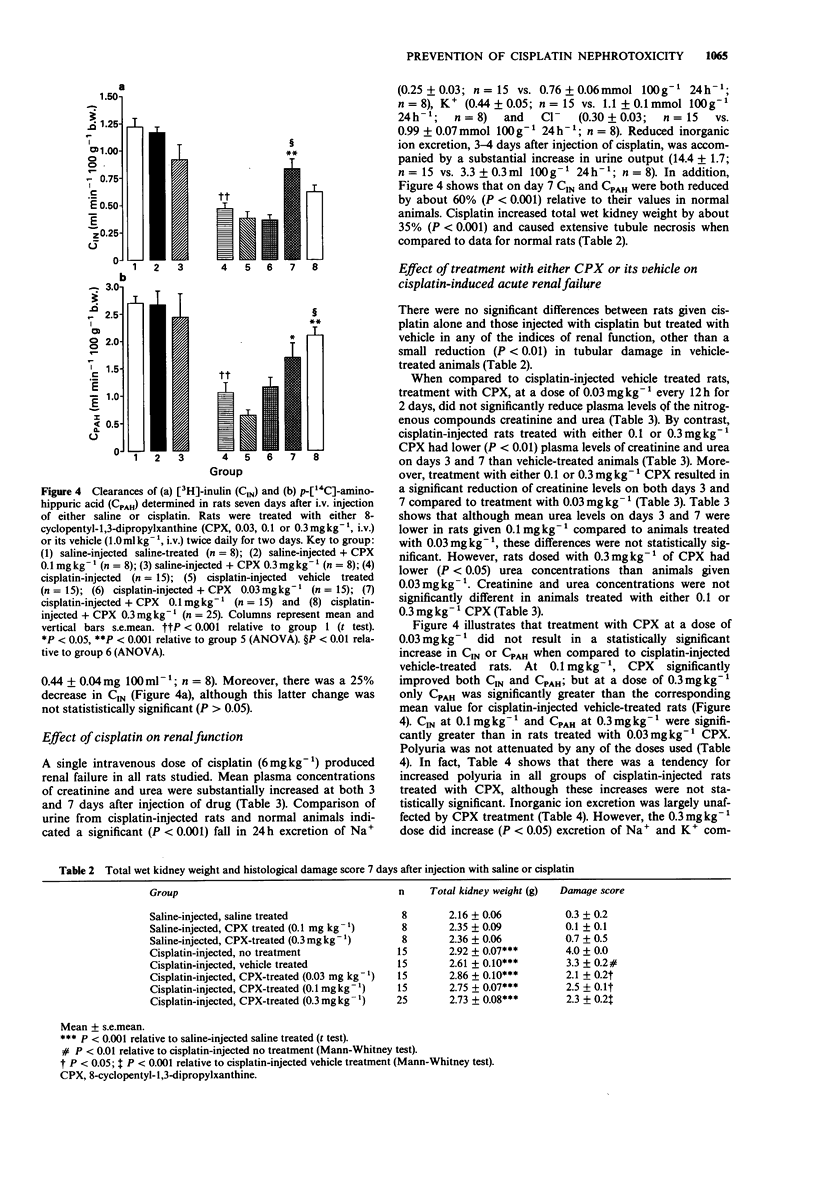
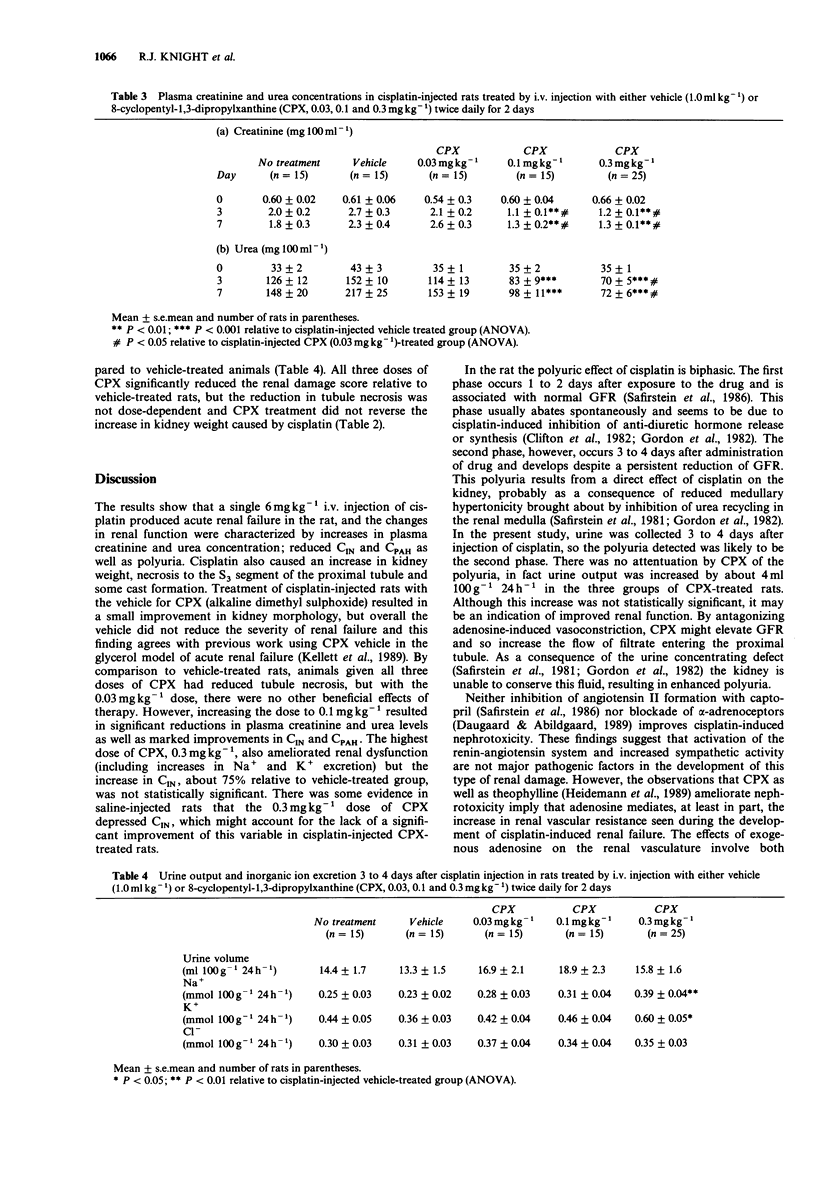
1. The effect of the selective adenosine A1-receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (CPX), on the development of cisplatin-induced acute renal failure was investigated in the rat. 2. CPX at doses of 0.03, 0.1 and 0.3 mg kg-1, i.v. caused increasing degrees of antagonism of adenosine-induced bradycardia in anaesthetized rats. The magnitude of antagonism was not directly proportional to the increment in dose, but for each dose, it was similar in rats injected with either saline or cisplatin. CPX at a dose of 0.03 mg kg-1 significantly antagonized adenosine-induced bradycardia for up to 2.5 h, while doses of 0.1 and 0.3 mg kg-1 produced significant blockade for periods longer than 5 h. 3. Administration of cisplatin (6 mg kg-1, i.v.) caused acute renal failure characterized by decreased inulin and p-aminohippurate clearances, increased urine volume but decreased excretion of Na+, K+ and Cl- ions and by increased plasma levels of urea and creatinine. Kidney weight was increased in cisplatin-treated rats and renal tubule necrosis occurred. 4. Administration of CPX (0.03 mg kg-1, i.v.; twice daily for two days) to rats given cisplatin did not reduce the severity of the resultant renal failure. However, treatment with 0.1 mg kg-1 CPX attenuated the increases in plasma creatinine/urea levels observed in rats on days 3 and 7 after induction of renal failure. In addition, this dose significantly reduced renal tubule damage and increased inulin and p-aminohippurate clearances. A similar pattern of protection was noted with CPX at a dose of 0.3 mg kg-1 although the increase in inulin clearance was not statistically significant.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Churchill P. C., Bidani A. K. Hypothesis: adenosine mediates hemodynamic changes in renal failure. Med Hypotheses. 1982 Mar;8(3):275–285. doi: 10.1016/0306-9877(82)90124-4. [DOI] [PubMed] [Google Scholar]
- Clifton G. G., Pearce C., O'Neill W. M., Jr, Wallin J. D. Early polyuria in the rat following single-dose cis-dichlorodiammineplatinum (II): effects on plasma vasopressin concentration and posterior pituitary function. J Lab Clin Med. 1982 Nov;100(5):659–670. [PubMed] [Google Scholar]
- Collis M. G., Keddie J. R., Torr S. R. Evidence that the positive inotropic effects of the alkylxanthines are not due to adenosine receptor blockade. Br J Pharmacol. 1984 Feb;81(2):401–407. doi: 10.1111/j.1476-5381.1984.tb10092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson R., Scheinman S. J. Xanthine effects on renal proximal tubular function and cyclic AMP metabolism. J Pharmacol Exp Ther. 1989 Feb;248(2):589–595. [PubMed] [Google Scholar]
- Daugaard G., Abildgaard U. Cisplatin nephrotoxicity. A review. Cancer Chemother Pharmacol. 1989;25(1):1–9. doi: 10.1007/BF00694330. [DOI] [PubMed] [Google Scholar]
- Daugaard G., Abildgaard U., Holstein-Rathlou N. H., Amtorp O., Leyssac P. P. Effect of cisplatin on renal haemodynamics and tubular function in the dog kidney. Int J Androl. 1987 Feb;10(1):347–351. doi: 10.1111/j.1365-2605.1987.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B. On the mechanism of action of theophylline and caffeine. Acta Med Scand. 1985;217(2):149–153. doi: 10.1111/j.0954-6820.1985.tb01650.x. [DOI] [PubMed] [Google Scholar]
- Gordon J. A., Peterson L. N., Anderson R. J. Water metabolism after cisplatin in the rat. Am J Physiol. 1982 Jul;243(1):F36–F43. doi: 10.1152/ajprenal.1982.243.1.F36. [DOI] [PubMed] [Google Scholar]
- Haleen S. J., Steffen R. P., Hamilton H. W. PD 116,948, a highly selective A1 adenosine receptor antagonist. Life Sci. 1987 Feb 9;40(6):555–561. doi: 10.1016/0024-3205(87)90369-9. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Guyton A. C., Farr B. M. A single-injection method for measuring glomerular filtration rate. Am J Physiol. 1977 Jan;232(1):F72–F76. doi: 10.1152/ajprenal.1977.232.1.F72. [DOI] [PubMed] [Google Scholar]
- Heidemann H. T., Müller S., Mertins L., Stepan G., Hoffmann K., Ohnhaus E. E. Effect of aminophylline on cisplatin nephrotoxicity in the rat. Br J Pharmacol. 1989 Jun;97(2):313–318. doi: 10.1111/j.1476-5381.1989.tb11956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz F. G., Steinhausen M. Renovascular effects of adenosine receptor agonists. Ren Physiol. 1987;10(5):272–282. doi: 10.1159/000173135. [DOI] [PubMed] [Google Scholar]
- Kellett R., Bowmer C. J., Collis M. G., Yates M. S. Amelioration of glycerol-induced acute renal failure in the rat with 8-cyclopentyl-1,3-dipropylxanthine. Br J Pharmacol. 1989 Nov;98(3):1066–1074. doi: 10.1111/j.1476-5381.1989.tb14639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. D., Churchill P. C. Effects of adenosine receptor agonists in the isolated, perfused rat kidney. Am J Physiol. 1984 Sep;247(3 Pt 2):H343–H348. doi: 10.1152/ajpheart.1984.247.3.H343. [DOI] [PubMed] [Google Scholar]
- Offerman J. J., Meijer S., Sleijfer D. T., Mulder N. H., Donker A. J., Koops H. S., van der Hem G. K. Acute effects of cis-diamminedichloroplatinum (CDDP) on renal function. Cancer Chemother Pharmacol. 1984;12(1):36–38. doi: 10.1007/BF00255906. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Ihde D. C., Linehan W. M., Jacob J., Ostchega Y., Young R. C. A randomized trial of standard chemotherapy v a high-dose chemotherapy regimen in the treatment of poor prognosis nonseminomatous germ-cell tumors. J Clin Oncol. 1988 Jun;6(6):1031–1040. doi: 10.1200/JCO.1988.6.6.1031. [DOI] [PubMed] [Google Scholar]
- ROSENBERG B., VANCAMP L., KRIGAS T. INHIBITION OF CELL DIVISION IN ESCHERICHIA COLI BY ELECTROLYSIS PRODUCTS FROM A PLATINUM ELECTRODE. Nature. 1965 Feb 13;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Safirstein R., Miller P., Dikman S., Lyman N., Shapiro C. Cisplatin nephrotoxicity in rats: defect in papillary hypertonicity. Am J Physiol. 1981 Aug;241(2):F175–F185. doi: 10.1152/ajprenal.1981.241.2.F175. [DOI] [PubMed] [Google Scholar]
- Safirstein R., Winston J., Goldstein M., Moel D., Dikman S., Guttenplan J. Cisplatin nephrotoxicity. Am J Kidney Dis. 1986 Nov;8(5):356–367. doi: 10.1016/s0272-6386(86)80111-1. [DOI] [PubMed] [Google Scholar]
- Samir Amer M., Kreighbaum W. E. Cyclic nucleotide phosphodiesterases: properties, activators, inhibitors, structure--activity relationships, and possible role in drug development. J Pharm Sci. 1975 Jan;64(1):1–37. doi: 10.1002/jps.2600640106. [DOI] [PubMed] [Google Scholar]
- Vidrio H., García-Márquez F., Magos G. A. Repeated administration of adenosine increases its cardiovascular effects in rats. Eur J Pharmacol. 1987 Jan 20;133(3):341–344. doi: 10.1016/0014-2999(87)90031-8. [DOI] [PubMed] [Google Scholar]
- Winston J. A., Safirstein R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am J Physiol. 1985 Oct;249(4 Pt 2):F490–F496. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]


